Introduction
The present incidence of MM in Korea has increased approximately 50 times compared with the 1980s. Additionally, developing new anticancer drugs (cf, lenalidomide, pomalidomide, ixazomib, elotuzumab, selinexor and daratumumab) as well as advanced clinical trial of combination with classic and new agents has enabled a wide range of treatment options and improvement in overall survival. In Korean we also experience improvement in MM treatment, however, there are limitations in applying new agent due to delay in approval and reimbursement in compared with US and EU. Thus, we try to analyze the outcome of mm treatment for 20 years and to find unmet need of myeloma treatment in Korea.
Methods
We have conducted a registry study for multiple myeloma patients since 2000 in Samsung Medical Center. At the time of analysis, 957 patients included in this study. We excluded amyloidosis, plasma cell leukemia and POEMS syndrome. And, we also excluded patients without enough data or short follow up due to transfer to other medical center.
Results
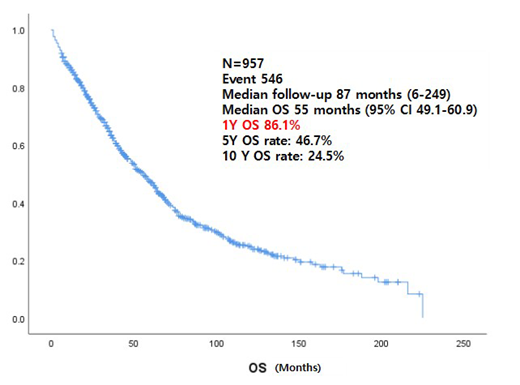
The median follows up duration were 87 months (range 6-249) and the median overall survival of total patients were 55 months (95% CI 49.1-60.9). Among the 957 patients, the median age was 61 (22-92), 582 (60.8%) of those were aged younger than 65 years and 375 (39.2%) of those were aged older than 65 years. The overall survival of younger than 65 years was longer than older than 65 years old (66 months, 95% CI 59.3-72.7 vs. 35 months, 95% CI 30.3-39.7). Over the course of the decades, the patients who enrolled during from 2010-2018(n=614) were more than those who enrolled during from 2000 to 2009 (n=343). There was no difference in overall survival between the two groups. The majority of patients (n=746, 78%) received bortezomib-containing regimens followed by lenalidomide containing regimens (n=373, 39%). Before transplantation, VAD (n=132), T(C)D(n=187) and VTD(n=158) were used as conditioning regimens. Among of them, VTD regimen showed a slightly improvement in PFS (22months, 95% CI 19.3-34.4) than T(C)D or VAD (19 months, 95% CI 15.0-23.0 vs. 22 months 95% CI 17.0-27.0, P-value <0.00) but, no difference in survival rates according to type of regimen (p-value=0.46). The induction chemotherapy of the newly diagnosed auto-HSCT ineligible patients (n=307) was CP/MP/CD/MD (n=103), VMP (n=172), LD (n=32). There was a difference in ORR (55.4%, 79.7%, 84.4%, P-value<0.00) and PFS (13 months vs. 16 months vs. 20 months, P-value <0.00) according to the regimen, but no difference in survival.
In present study, 489 patients (51.1%) received hematopoietic stem cell transplantation (HSCT), of which 403 (82.4%) patients received single auto-HSCT and 45 (9.2%) patients received tandem auto-HSCT. The survival of patients with auto-HSCT was 34 months (95% CI 29.0-39.0) in patients who were not treated with 75 months (95% CI 64.4-85.6, p-value < 0.00). The survival rate of patient who experienced second recurrence (n=613) was 29 months (95% CI 24.3-33.7), and those of the third recurrence (n=424) was 20 months (95% CI 16.1-23.9). Of the 239 patients who recurred after bortezomib and lenalidomide, 118 patients had received new agent including pomalidomide(n=109), carfilzomib(n=50), daratumumab and isatuximab (n=30). The overall survival of these patients was longer than not taking the new agents. (73 months, 95% CI 61.8-84.2 vs. 45 months, 95% CI 31.5-58.5, p-value<0.00).
Conclusion
In spite of shortcoming as a single center data, long duration and relatively large number we could see the overall outcome of MM treatment in the era of novel agent. We could see the improvement of outcome who experience novel drug treatment. However, we could not see overall survival improvement from the patients who were diagnosed later 10 years. We suspect that even the earlier patients experienced survival improvement due to salvage treatment of bortezomib and lenalidomide, but the exposure of second line novel drug is minimal for the latter group patients. In conclusion, we improved the outcome of MM treatment in Korea with novel drugs for past 20 years, but still we have unmet need to improve survival of MM patients and need to more active use of novel agent for MM patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.