Introduction: Liposomal daunorubicin/cytarabine (Vyxeos®) is a dual drug liposomal encapsulation of cytarabine and daunorubicin, delivering drugs at a fixed 5:1 synergistic ratio for a longer therapeutic period. Compared to standard 7+3, liposomal cytarabine/daunorubicin (lipo-cytara/dauno) patients had improved survival and remission rates in a pivotal phase III study of elder adults with high-risk acute myelogenous leukemia (AML). Furthermore, more lipo-cytara/dauno patients went to allogeneic stem cell transplantation (HCT), with lower mortality and improved survival compared to those induced with 7+3. With its enhanced pharmacokinetics, lipo-cytara/dauno may provide a potent bridge to transplant. We report our experience using lipo-cytara/dauno as a bridge to same donor lymphocyte infusion (DLI) or different donor HCT in high-risk AML.
Methods: We retrospectively reviewed all patients who received lipo-cytara/dauno at our institution since the FDA approval in August 2017. Of the 21 patients who have been treated, 9 received it as a bridge to cell therapy. All patients received the drug by usual means under the FDA label.
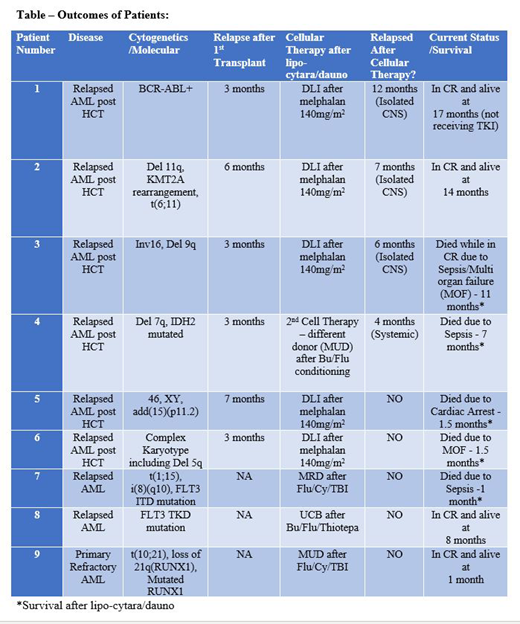
Results: The median age of the 9 patients who received lipo-cytara/dauno as a bridge to cell therapy was 59 years. Seven were male, and two were female. Patients had had 1-4 prior lines of chemotherapy (median 2) with 7 of 9 patients having received prior standard 7+3 induction (cytarabine 100-200 mg/m2 x 7 days infusion and daunorubicin 60-90 mg/m2 x 3 days). Most had adverse cytogenetics, and all 9 patients received full lipo-cytara/dauno induction (daunorubicin 44 mg/m2 and cytarabine 100 mg/m2 on Days 1, 3, and 5) as outpatient therapy [Table].
Of the 9 patients, 6 had AML with very early relapse after HCT, with median time to relapse of 4 months (range 3 to 7 months). All 6 successfully proceeded to their planned cell infusion: 5 received same donor DLI after melphalan at 140 mg/m2, and 1 underwent second HCT from a different donor with busulfan/fludarabine conditioning at Days 15-40 after lipo-cytara/dauno.
Of the remaining 3, two had relapsed AML after an initial remission (one was in first complete remission (CR1) for 3 months after 7+3/midostaurin induction and the other was in CR1 for 7 months after 7+3 induction/high dose cytarabine consolidation) and one had primary refractory disease (PREF) after 7+3 and azacitidine/venetoclax induction regimens. All 3 successfully underwent first HCT at Days 15-100 days after lipo-cytara/dauno bridge. The PREF patient received fludarabine/cyclophosphamide/TBI conditioning followed by matched unrelated donor transplant. Of the 2 with relapsed AML after initial remission, one received busulfan/fludarabine/thiotepa conditioning followed by umbilical cord stem cell transplantation and the other patient received fludarabine/cyclophosphamide/TBI conditioning prior to matched related donor transplant.
Six of 9 had Day 14 bone marrow biopsies after lipo-cytara/dauno: 2 were in CR, 2 had >80% cytoreduction, and 2 had similar blast count. Three with persistent disease underwent reinduction with lipo-cytara/dauno (Days 1 and 3) and proceeded straight to cell therapy after. Median days to hospitalization after outpatient lipo-cytara/dauno was 6 days (range 3 to 14 days).
Four out of 9 patients remain alive. Two were very early post HCT relapses (relapsed at 3 and 6 months post-HCT), both of which are remarkably in CR at 14 and 17 months after second cell therapy. Interestingly, both had CNS relapse, which were successfully treated, and both remain alive and in remission today. The other two had relapsed AML and PREF AML and underwent first HCT after lipo-cytara/dauno bridge. They remain alive and in remission at 1 and 8 months.
Conclusion: In this retrospective study, outpatient lipo-cytara/dauno as a bridge to cell therapy is feasible and effective in very high-risk AML with no other viable options. While preliminary, survival appears favorable to that reported elsewhere at 14-23% at 1 year in this poor risk group, including those with adverse cytogenetic and/or very early post-HCT relapse. Prospective multi-center trials are planned to further evaluate lipo-cytara/dauno as a bridge to DLI/HCT in those with early relapse post-HCT and in those with refractory disease, with therapy to include CNS prophylaxis.
Stiff:Gilead/Kite Pharma: Consultancy, Honoraria, Research Funding; Amgen: Research Funding; Gamida-Cell: Research Funding; Incyte: Research Funding; Cellectar: Research Funding; Unum: Research Funding. Tsai:Jazz pharmaceuticals: Speakers Bureau; Jazz pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.