Introduction
Emerging research suggests key differences in clinical manifestations among people with hemophilia A (HA) and hemophilia B (HB) that may impact health-related quality of life (HRQoL), healthcare utilization and costs. However, HB's low prevalence hinders obtaining study cohorts large enough to be representative and avoid selection bias. This analysis of the Hematology Utilization Group Studies (HUGS) cohorts examined over a 2-year period (1) baseline pain, joint range of motion (ROM), and HRQoL (2) clinical characteristics and treatment outcomes, and (3) costs of care and service utilization among a geographically diverse sample of individuals with HB and HA.
Materials and Methods
HUGS part Va (enrolling HA) and HUGS part Vb (enrolling HB) are US multicenter observational studies conducted at hemophilia treatment centers (HTCs) serving patients from 15 states. HUGS Va was conducted 2005-2007; HUGS Vb 2009-2014. This analysis included 350 participants with complete medical records and ≥2 follow-up surveys: 243 with HA and 107 with HB. Children (age 2-17 years) and adults (age 18-64 years) were followed prospectively. Mean ages at baseline were 21.3 (HA) and 24.5 (HB); 70% of HA participants were severely affected, as were 46% with HB. HUGS collected data through an initial, in-person interview with participants or parents after informed consent; regularly scheduled web-, mail-, or phone-based follow-up questionnaires; and clinical chart review conducted by HTC staff. Treatment utilization and costs were annualized including direct and indirect costs. Medication cost was obtained from payment allowance limits for Medicare Part B. Costs were adjusted for inflation to reflect costs in 2019. We compared continuous variables using Wilcoxon-Mann-Whitney and Chi-square or Fisher's exact test for categorical variables.
Results
Quality of Life and Clinical Measures
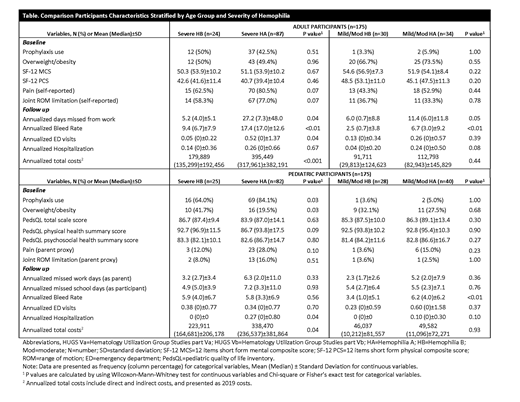
Both severe adults HA (mean±SD: 40.7±10.4) and HB (42.6±11.4) showed lower SF-12 physical composite score than the general US population (50±10). Severe HB children had mean 6 points greater physical health summary score than severe HA children. Pain and joint ROM limitation were reported less often by those with severe HB (63% and 58%, respectively), compared to those with severe HA (81% and 77% respectively). Among HB children with severe hemophilia, 12% reported pain compared to 28% with HA. Joint ROM limitation was reported in 8% of HB children, compared to 16% with HA. HB children having half the burden than HA on reported pain and joint ROM limitation. Median annualized missed work days were significant lower in the HB group (3) than HA (7), P<0.01. Adults with severe HB missed a median 4 days of work annually, compared to 7 days for those with severe HA (P=0.04). At baseline, the pediatric severe HB group had overweight/obesity double the HA group (P=0.03), but nearly 50% of both adult severe populations were overweight/obese.
Treatment and Outcomes
At baseline, adults with severe HB were slightly more likely to use prophylaxis than HA adults (50% versus 43%). HA children, however, were more likely than HB children (84% versus 64%) to treat prophylactically (P=0.03). Mild/moderate HA and HB cohorts rarely used prophylaxis. At follow-up, both severe and mild/moderate HA adults experienced significantly greater annualized bleeding rates than adults (P<0.01).
Cost and Service Utilization
The follow-up data indicated that the median number of ED visits among both severe HA and HB adults was 0, but the means were 0.1 and 0.5 respectively (P=0.04), and range was lower among the HB group (0-1) than in HA (0-8). Among adults, median annual total costs for severe HB ($135,299) were significantly lower than for severe HA ($317,961), P<0.001. Among children, median annual total costs among those with severe HB ($164,681) were lower than for those with severe HA ($236,537), P=0.04.
Conclusions
At baseline, the HUGS Va and Vb cohorts revealed less clinical burden among those with HB than those with HA, indicating that people with HB may experience fewer symptoms and effects from the disease. The fact that the HB cohort had lower costs and healthcare utilization over a 2-year period supports this finding. Overweight/obesity exceeded 2/3 for adults with mild/moderate HA and HB. Despite the relatively small HB sample size, analyses of HUGS cohort studies provide valuable information about people treated at HTCs in the US.
Curtis:Wyeth, now Pfizer: Consultancy; Bayer Foundation: Consultancy; CSL Behring: Consultancy; Baxalta, now part of Takeda: Consultancy; Novo Nordisk: Consultancy. Lou:Wyeth, now Pfizer: Research Funding. Tran:Pfizer: Honoraria; Bioverativ: Honoraria; Novo Nordisk: Honoraria; Bayer: Honoraria. Wu:Bayer Foundation: Research Funding; Novo Nordisk: Research Funding; Baxalta, now part of Takeda: Research Funding; CSL Behring: Research Funding; Wyeth, now Pfizer: Research Funding. Nichol:Novo Nordisk: Research Funding; CSL Behring: Research Funding; Wyeth, now Pfizer: Research Funding; Bayer Foundation: Research Funding; Baxalta, now part of Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.