Introduction: Anemia occurs in 80-90% of patients with myelodysplastic syndromes (MDS), nearly half of whom are red blood cell (RBC) transfusion dependent (TD) at diagnosis. The presence of anemia, transfusion dependency, and the resulting complications exacerbate the burden of MDS. Patients with lower-risk MDS have longer overall survival (OS) than patients with higher-risk MDS; however, TD status and higher transfusion load significantly reduce OS. Treatment of lower-risk TD MDS aims to achieve RBC transfusion independence (TI), hematologic improvements and alterations in erythroid response according to IWG 2006 criteria, and improved health-related quality of life (HRQoL). To evaluate these points, we performed a systematic literature review (SLR) to assess the burden experienced by adult patients with RBC TD anemia in lower-risk MDS.
Methods: Embase®, MEDLINE®, and the Cochrane Library were systematically searched from inception to March 2019 to identify observational studies reporting on the HRQoL, clinical, and epidemiological burden as well as treatment patterns for adult patients with lower-risk MDS who were RBC TD. Conference proceedings from the last 2 years were searched to identify recent data not yet published as a full text article. Only English language articles and conference abstracts were included. References were screened at the title/abstract and full-text levels by 2 independent reviewers, with methodology following Cochrane guidelines.
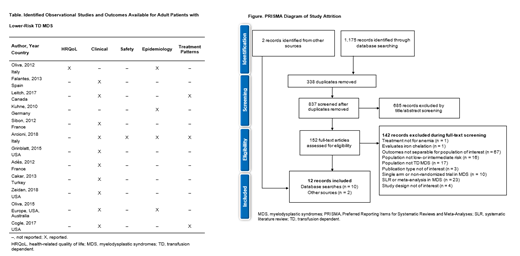
Results: Database searches yielded 1,175 records. After removing duplicates, 837 abstracts were reviewed, with 152 publications progressing to full-text review. Ten studies met the eligibility criteria. Two additional records were identified from hand searching (Figure). Many studies reported on mixed MDS patient populations, where only a subset were lower-risk and TD, limiting the amount of data available. Nine studies were from the USA or EU5 (France Germany, Italy, Spain, United Kingdom) as well as 1 international study encompassing Europe, the USA, and Australia. The majority of studies defined risk status using the International Prognostic Scoring System (IPSS) risk score, with only 1 study reporting both IPSS and Revised-IPSS (IPSS-R) score. Where reported, patients were mostly older (mean age > 70 years). Burden data were sparse, with few outcomes reported across multiple studies (Table). One study from Italy (mean age 72 years) reporting EuroQoL 5-dimension Visual Analogue Scale data indicated that patients with TD MDS had worse scores (53.0) compared with patients aged 65-74 years in the general Italian population (67.8). Clinical data indicated a variable OS rate across studies conducted in Spain and France, as well as in the 1 international study, with a 2-year OS rate of 73.2% and 4-year OS rates ranging from 27.6% to 73%. The data consistently reported that patients with TD MDS had shorter OS than patients with TI MDS. Differences in timepoints for assessment, types of treatments, and RBC transfusion load increased the variation in OS data. TI was defined as a transfusion-free period of ≥ 56 days, or 8 weeks, in most studies. Median time to reach TI (when reported) ranged from 32-97 weeks, although the prolonged length of time it took to reach TI and the proportion of patients achieving TI indicated an extensive time on treatment. Similar conclusions were drawn in 3 studies that compared OS in patients who became TI with those who remained TD. Epidemiology data were limited to mortality data, and no data were available on morbidity, incidence, or prevalence of disease. Mortality data provided an incomplete picture; deaths were reported as overall mortality or non-leukemic deaths, obscuring mortality rates specifically associated with lower-risk TD MDS. Treatment patterns were reported in 3 studies; erythropoiesis-stimulating agents and hypomethylating agents were the most common treatments reported.
Conclusions: Data on the burden of illness in patients with lower-risk MDS with TD anemia were limited and reported inconsistently across studies. Despite this heterogeneity, HRQoL outcomes had a trend toward worse scores in patients with TD MDS than in the general population. OS rate at 2 and 4 years indicated worse prognosis in patients with TD MDS compared with the general population. To better understand the burden of illness, further robust observational studies are needed to close the evidence gaps in this population.
Deshpande:Evidera: Employment; Celgene Corporation: Consultancy. Turner:Evidera: Employment; Celgene Corporation: Consultancy. Byrnes:Evidera: Employment. Pelligra:Celgene Corporation: Research Funding; Evidera: Employment. Rines-MacEachern:Evidera: Employment. Chitnis:Celgene Corporation: Consultancy; Evidera: Employment. Kulasekararaj:Celgene Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Achilleon: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Ra Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Akari Therapeutics: Consultancy. Tang:Celgene Corporation: Employment, Equity Ownership. Morison:Celgene Corporation: Employment. Oliva:Apellis: Consultancy; Celgene Corporation: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.