Background: Reproductive-age women are at increased risk of venous thromboembolism (VTE) due to increased estrogen states, such as pregnancy and the use of estrogen-based medications. The use of oral anticoagulants (OACs), the standard of care for VTE, is associated with increased rates of heavy menstrual bleeding (HMB), defined as >80 mL loss of blood per cycle, in this patient population. In addition to a need for interventions, ranging from initiation of hormonal therapy to surgical management of bleeding, HMB is a deterrent for adherence to OAC. Decreased adherence, such as temporary discontinuation, is associated with an increased risk of recurrent VTE. This is particularly true for women treated with rivaroxaban, for whom the literature suggests the risk of HMB and other forms of abnormal uterine bleeding is increased two-fold, as compared to warfarin.
Methods: We performed a retrospective cohort study of reproductive-aged (18-50 years) women receiving oral anticoagulant therapy at a single tertiary care center between January 1, 2012 and December 31, 2018. Female subjects within the appropriate age range were identified using a Cohort Discovery Tool, a web-based interface which allows investigators to query an underlying research data warehouse that contains electronic health record data for the Oregon Health & Science University healthcare system. Medical record review was performed on all identified records to confirm inclusion criteria, including a documented visit with a primary care provider within 6 months following initial prescription. Exclusions included surgical menopause, pregnancy within 6 months of initial prescription, and lactation within 3 months. We abstracted demographic information, initial anticoagulant prescription and indication, menstrual bleeding history, hormonal therapy and HMB specific medical or surgical treatment within 6 months of initial prescription.
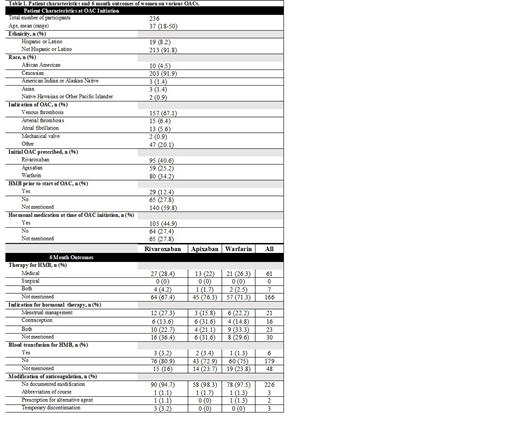
Results: A total of 236 eligible subjects were identified. The majority (40.6%) were prescribed rivaroxaban as compared to apixaban (25.2%) or warfarin (34.2%). Most (67.1%) were receiving anticoagulation for the treatment of VTE although 20.1% were anticoagulated for 'other' reasons, including superficial thrombophlebitis and prophylaxis in the post-operative setting or for travel. For the majority of subjects (59.8%), no discussion of menstrual history was documented prior to or at the time of initial prescription. Twenty-nine percent of women received medical (including hormonal) or surgical therapy for HMB, including 32.6% of women treated with rivaroxaban, 23.7% of women treated with apixaban and 28.8% of women treated with warfarin (p <0.001).
Discussion: Our study confirms prior reports of increased prevalence of HMB and other abnormal uterine bleeding in women prescribed rivaroxaban as compared to apixaban or warfarin. Our overall numbers, however, are lower than those previously reported in the literature, where HMB, defined as blood loss of >80mL/cycle or a pictorial blood loss assessment chart score of >100, is reported in 66% of warfarin users. We used a different definition of HMB, which was any menstrual bleeding severe enough to require an intervention, including hormonal therapy, surgery or iron replacement, which may explain this difference. Not all women with standardly defined HMB may require such therapies. However, since the vast majority of providers did not assess for menstrual bleeding at the time of drug initiation, it is possible that HMB was unrecognized. Our next step will be to survey women who received an initial prescription in 2017 or 2018 regarding symptoms of HMB, in order to assess the proportion of subjects whose diagnosis of HMB went undetected. Our findings underscore the importance of addressing and discussing menstrual bleeding with menstruating women using oral anticoagulants.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.