Autologous hematopoietic stem cell transplantation (Auto-HSCT) with gene-modification techniques represents a potential cure for multiple genetic blood diseases. Despite its broad curative potential, auto-gene modified HSCT is currently limited due to morbidity/mortality from cytotoxic chemotherapy-based conditioning, including risks of secondary malignancies, organ toxicity, and infertility. To overcome these limitations, we have developed antibody drug conjugates (ADC) targeting CD117 (C-KIT) to specifically deplete the hematopoietic stem and progenitor cells (HSPC) prior to auto-gene modified HSCT. We have previously shown that the anti-CD117 ADC is highly effective at killing human CD117+ cells in vitro and in vivo (Pearse et al., Blood 2018 132:3314). To validate CD117 as an appropriate antigen for targeted ADC-mediated depletion prior to HSCT, we developed an optimized non-human primate (NHP) tool anti-CD117 ADC and evaluated it in an auto-gene modified HSCT in the rhesus macaque model.
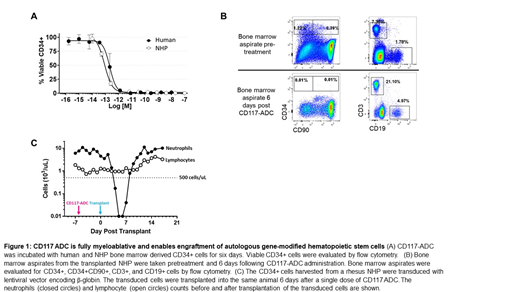
The tool CD117-ADC is potent on primary human and NHP CD34+ cells in vitro with EC50 of 0.2 and 0.09 pM respectively (Figure 1A). Humanized NSG mice treated with the tool CD117-ADC had full depletion of human HSPCs in the bone marrow 21 days after a single administration of the ADC, while maintaining the peripheral immune cells. We next tested the efficacy and safety of the tool CD117-ADC in NHPs. A single administration of the tool CD117-ADC was fully myeloablative (>99% HSPC depletion) and comparable to HSPC depletion observed following busulfan conditioning (6 mg/kg, once daily for 4 consecutive days). There was no effect on the peripheral and bone marrow lymphocytes and the ADC was well tolerated. To facilitate the use in HSCT, the tool CD117-ADC was engineered to have a fast clearance and in this study the half-life was <10 hours.
Based on these encouraging results, we explored whether the tool CD117-ADC could enable engraftment of autologous gene modified hematopoietic stem cells in the rhesus macaque model. A single rhesus macaque was mobilized with granulocyte-colony stimulating factor (G-CSF, 20 mcg/kg/day x 5) and plerixafor (1 mg/kg on day 5 of G-CSF) prior to apheresis. The isolated CD34+ cells were transduced with a lentivirus encoding the β-globin gene and cryopreserved. The tool CD117-ADC was dosed on day -6 and the cryopreserved gene modified cells were thawed and infused (3.3 x 106 CD34+ cells/kg) on day 0. A bone marrow aspirate analyzed on the day of infusion (day 0) demonstrated >99% depletion of the HSPCs and preserved of the bone marrow lymphocytes (Figure 1B). The primate engrafted neutrophils and platelets on day 8 and 10 respectively, and the peripheral lymphocytes were maintained throughout the transplant (Figure 1C). The gene marking in the granulocytes was detectable at day 9, and additional follow up and data from additional animals will be presented.
In summary, we have developed a tool CD117 ADC that shows potent activity on NHP CD34+ cells. This optimized CD117-ADC is fully myeloablative with a single dose in NHPs, has a favorable safety profile, spares the immune system and is cleared rapidly as designed. In a rhesus model of autologous gene modified HSCT, a single dose of the ADC enables engraftment of auto-gene modified HSC. These proof of concept studies validate the use of CD117-ADC for targeted HSPC depletion prior to transplant and support its use as a new conditioning agent for autologous gene modified HSCT. This targeted approach for safer conditioning could improve the risk benefit profile for patients undergoing stem cell transplant and enable more patients to benefit from these potentially curative therapies.
Pearse:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties. McDonough:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties. Proctor:Magenta Therapeutics: Employment, Equity Ownership. Panwar:Magenta Therapeutics: Employment, Equity Ownership. Sarma:Magenta Therapeutics: Employment, Equity Ownership. Kien:Magenta Therapeutics: Employment, Equity Ownership. Latimer:Magenta Therapeutics: Employment, Equity Ownership. Dushime:Magenta Therapeutics: Employment, Equity Ownership. Hyzy:Magenta Therapeutics: Employment, Equity Ownership. Brooks:Magenta Therapeutics: Employment, Equity Ownership. Palchaudhuri:Magenta Therapeutics: Employment, Equity Ownership. Li:Magenta Therapeutics: Employment, Equity Ownership. Sawant:Magenta Therapeutics: Employment, Equity Ownership. McDonagh:Magenta Therapeutics: Employment. Boitano:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties. Cooke:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.