Background:
Most data on overall survival (OS) and adverse events (AEs) in patients with mantle cell lymphoma (MCL) are from controlled trials in academic centers; data from real world management and outcomes in patients with MCL are sparse. We therefore conducted a population-based retrospective cohort study of patients with MCL in the Medicare database to assess treatment patterns, OS, AEs, and economic burden.
Methods:
Patients with MCL who received any systemic cancer-directed treatment from 2013 to 2015 were selected from the nationwide Medicare claims database and followed through 2016. The date of the first observed systemic therapy defined each patient's index date. Patients were included if they (a) were ≥ 18 years of age at the index date; (b) had ≥ 12 months of continuous Medicare enrollment before the index date (baseline period); and (c) had no evidence of prior MCL-directed treatment (systemic therapy and/or SCT) at any time before the index date (i.e., during at least the previous 12 months). An observed line of therapy was defined as all agents received on or within 35 days after the first claim for a systemic therapy drug; the observed therapy line was considered ended upon switch to another regimen or a gap ≥ 90 days after the last treatment. OS was estimated by the Kaplan-Meier method from the index date (start of first observed line of therapy) until the last follow-up or death. We also calculated rates of occurrence for hematologic and nonhematologic AEs often associated with the most commonly observed regimens (irrespective of observed line of therapy). The occurrence of AEs was defined based on the presence of at least one claim containing an AE-specific diagnosis code during the treatment, regardless of any history of the AE before treatment initiation. All-cause health care costs were assessed from Medicare's perspective. Multivariable models were fitted to assess the association between number of AEs and average costs during the first observed therapy.
Results:
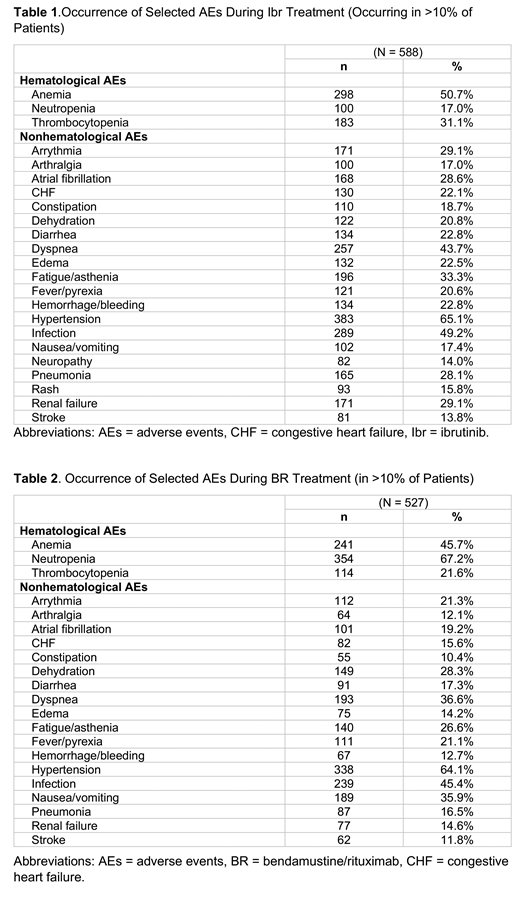
We analyzed 1,465 patients who met the inclusion criteria (median age=74 years; 68% male; 93% white). Across all observed lines of therapy, ibrutinib monotherapy (Ibr) (n=588 [40%]) was the most frequently used regimen, followed by bendamustine/rituximab (BR) (n=527 [36%]). Ibr recipients had a median age of 75 years, median Charlson Comorbidity Index (CCI) score of 4.0, and were followed for a median duration of 15 months; 52% died during the study period. BR recipients had a median age of 75 years, median CCI score of 3.0, and were followed for a median duration of 21 months; 28% died during the study period. In Ibr recipients, median OS was 22 months (95% CI = 16.9-28.6) and 24-month OS was 47% (95% CI = 42.9%-50.5%). In BR recipients, median OS was not reached while OS at 24 months was 73% (95% CI = 69.4%-76.0%). The occurrence of common AEs during Ibr and BR therapies are presented in Tables 1 and 2. The average per patient per month costs, among all patients, were $2,501 (SD = $2,818) during the baseline period and $12,604 (SD = $14,437) during the period after initiation of the first observed MCL-directed systemic therapy. Multivariable analysis showed that the patients with 3 or more AEs had nearly 4 times higher monthly per patient costs (cost ratio = 4.12, 95% CI = 3.53-4.82) compared with those with 0-2 AEs.
Conclusions:
Two-year survival rates observed in this study are comparable to those reported in clinical trials (47% for Ibr in the relapsed disease setting [Wang, 2015, Blood]) and nearly 75% for BR in patients with relapsed indolent disease and MCL [Rummel, 2016, Lancet]). Rates of AE occurrence in Ibr- and BR-treated patients in this study highlight the substantial burden and susceptibility to AEs among Medicare patients in the real-world setting. These findings also demonstrate a substantial increase in the economic burden from the baseline period to the period after MCL treatment initiation and as the number of AEs increased.
Kabadi:AstraZeneca: Employment, Equity Ownership. Goyal:RTI Health Solutions: Employment. Nagar:RTI Health Solutions: Employment. Davis:RTI Health Solutions: Employment. Le:AstraZeneca: Employment, Other: Stocks. Wang:Dava Oncology: Honoraria; Guidepoint Global: Consultancy; BioInvent: Consultancy, Research Funding; VelosBio: Research Funding; Loxo Oncology: Research Funding; Celgene: Honoraria, Research Funding; Juno Therapeutics: Research Funding; Aviara: Research Funding; Kite Pharma: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pharmacyclics: Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; MoreHealth: Consultancy, Equity Ownership; Acerta Pharma: Consultancy, Research Funding. Kaye:RTI Health Solutions: Employment.
Author notes
Asterisk with author names denotes non-ASH members.