Introduction:
CAR T-cell therapy has revolutionized treatment for patients with relapsed/refractory (r/r) diffuse large b-cell lymphoma (DLBCL). Although impressive durable responses can be achieved, this is weighted by adverse events including neurotoxicity and cytokine release syndrome (CRS). Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI) is a validated score that utilizes comorbidities to predict outcomes in patients receiving allogeneic stem cells transplants, but it not validated in CAR T-cell recipients. Meanwhile, the Cumulative Illness Rating Scale (CIRS) is a comprehensive tool which has been incorporated into clinical research in lymphoma. Here we report the impact of comorbidities, as measured by the HCT-CI and CIRS, on survival and tolerance of CAR T-cell therapy in patients with r/r DLBCL treated off clinical protocols.
Methods:
We conducted a retrospective analysis of patients with r/r DLBCL treated with commercial CART product at 4 academic medical centers after approval by the respective institutional review boards. Comorbidity data was assessed at the time of T-cell collection, and outcome data was assessed per last known follow-up. CIRS score was calculated as in Salvi et al, 2008. HCT-CI score was calculated as in Sorror et al, 2005. High comorbidity burden was defined as CIRS score ≥7, presence of severe impairment (CIRS score of 3 or 4 in ≥1 organ system; CIRS-3+), or HCT-CI ≥3. Cox proportional hazards models adjusted for age, ECOG performance status, number of prior therapies, type of product, cell of origin, MYC positivity on FISH, and comorbidities were used to assess effect on progression free survival (PFS) and overall survival (OS).
Results:
We analyzed 59 patients, with a median age of 63 years (range, 25-82). 80% of patients had an ECOG PS of 0 or 1. Median number of prior therapies was 3 (range, 2-6). 15 patients received tisagenlecleucel, and 44 patients received axicabtagene ciloleucel. 30 patients had germinal center B-cell (GCB) subtype DLBCL, and 29 were non-GCB as determined by Hahn's algorithm. 13 patients exhibited a MYC rearrangement by FISH, of which 9 also had BCL2 or BCL6 chromosomal translocation. Four patients did not receive CAR T-cells (3 died of disease progression; 1 died of an unrelated event). All patients (N=59) were used in the analysis. Median total CIRS was 10 (range, 1-20). 40 patients had score ≥7 and 29 had a CIRS-3+. 16 patients had neither total CIRS score ≥7 or CIRS-3+. The most common comorbidities were seen in the upper GI, endocrine and vascular/hematopoietic systems. Median HCT-CI was 2 (range, 0-13). Median follow-up was 154 days.
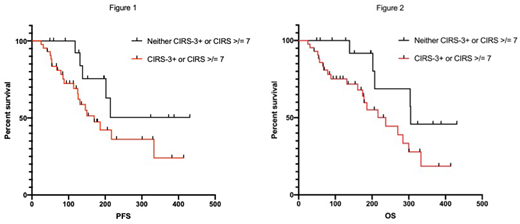
Significant comorbidities assessed by CIRS (either total score ≥7 or CIRS-3+) were associated with inferior PFS (HR 3.65, p=0.037) and OS (OS; HR 5.31, p=0.025) compared to patients without significant comorbidities in univariate analyses. Median PFS was not reached, and OS was 305 days for patients without significant comorbidities, and was 170 and 237 for patients with significant comorbidities respectively (Figure 1,2). HCT-CI ≥3 was not predictive of PFS (HR 0.85, p=0.70) or OS (HR 1.06, p=0.90). MYC positivity was associated with inferior OS (HR 2.64, p=0.033). Presence of comorbidities assessed by CIRS and HCT-CI, were not associated with incidence of neurotoxicity or CRS.
Presence of comorbidities by CIRS retained independent significance in multivariate models of PFS (HR 8.64, p=0.002) and OS (HR 15.44, p=0.042) when adjusted for all covariates. MYC also retained independent significance regarding OS (HR 3.23, p=0.032). Interestingly, older age was associated with superior PFS (HR 0.54, p=0.001) and OS (HR 0.59, p=0.007).
Conclusions:
In this multicenter retrospective analysis we show that CIRS has prognostic significance in patients with r/r DLBCL treated with commercial CAR T-cell therapy, as increased comorbidities, defined by presence of total CIRS score ≥7 or CIRS-3+, were associated with both a shorter PFS and OS. We did not find an association between the HCT-CI score and survival, nor did we see an association between comorbidities and incidence of neurotoxicity or CRS, however numbers were small. These findings show that evaluating comorbidities in patients eligible to receive CAR T-cell therapy should be considered. Further validation is needed to determine the extent that patient comorbidities predict survival in DLBCL patients undergoing CAR-T therapy.
Bishop:Novartis Pharmaceuticals: Consultancy. Stephens:Karyopharm: Research Funding; Gilead: Research Funding; Acerta: Research Funding. Hill:Amgen: Research Funding; TG therapeutics: Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celegene: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria; Takeda: Research Funding; Kite: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Research Funding; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Honoraria. Danilov:Seattle Genetics: Consultancy; MEI: Research Funding; Genentech: Consultancy, Research Funding; TG Therapeutics: Consultancy; Verastem Oncology: Consultancy, Other: Travel Reimbursement , Research Funding; Bayer Oncology: Consultancy, Research Funding; Pharmacyclics: Consultancy; Aptose Biosciences: Research Funding; Janssen: Consultancy; Celgene: Consultancy; Curis: Consultancy; Abbvie: Consultancy; AstraZeneca: Consultancy, Research Funding; Gilead Sciences: Consultancy, Research Funding; Takeda Oncology: Research Funding; Bristol-Meyers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.