Background: CD8+ T-cells in AML pts co-express multiple inhibitory receptors (IRs), including PD1, and IR expression increases with disease progression (Knaus et al, JCI Insight 2018). AZA upregulates pathways related to immunity and immune evasion in tumor cells, including PD-L1, (Wrangle et al, Oncotarget 2013) providing rationale for exploring AZA/Pembro combination in AML.
Aims: To assess safety and response to AZA/Pembro after minimum 2 cycles of therapy in relapsed/refractory (R/R) (Cohort 1) and newly diagnosed (dx) older AML (Cohort 2).
Methods:Cohort 1: Pts must have failed prior AML therapy. The first 6 pts (run in phase) received AZA 75 mg/m2 Days (D) 1-7 with Pembro 200 mg beginning on D8 and every (q)3 weeks (wks) thereafter. AZA cycles were repeated q4wks. No pts experienced dose limiting toxicity after minimum 3 cycles observation. After safety was established with the dosing schedule, patients with prior allogeneic stem cell transplant (alloSCT) were included and Cohort 2 started enrollment.
Cohort 2: Pts ≥65 years (yrs) with newly dx AML and not candidates, or unwilling to receive, intensive chemotherapy.
Other eligibility criteria (Cohort 1 and 2): ECOG PS 0-2 (changed to PS 0-1), adequate organ function, and no autoimmune processes requiring systemic immunosuppression.
Results:
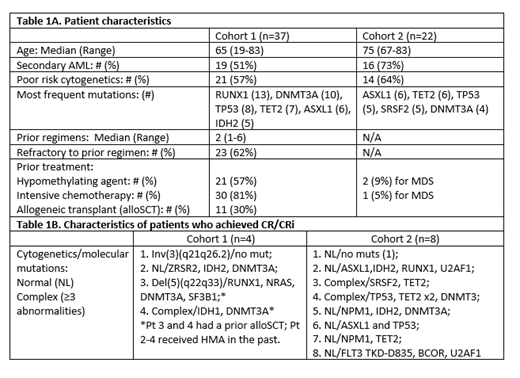
Efficacy: Cohort 1: 37 R/R pts have been enrolled. Baseline characteristics are summarized in Table 1A. 29 (78%) pts completed at least 2 cycles and are evaluable for response: 4 achieved complete remission (CR)/CR with incomplete hematologic recovery (CRi) (2/2) (14%) (Table 1B), 1 partial remission (PR) (4%), 4 hematologic improvement (HI) (14%), and 7 stable disease (SD) for at least 6 cycles (24%). The median # of cycles to response was 4 (range, 2-6). The 4- and 8-week mortality were 8% [all with rapidly progressive disease (PD): 2 received AZA for 3 and 5 days only] and 13%, respectively. With a median follow-up of 14.9 months (mos), the median overall survival (OS) for the whole cohort, responders + SD, and CR/CRi/PR is 10.8 mos (40% 1-yr), 13.9 mos (51% 1-yr), and 17.2 mos (75% 1-yr). The median event-free survival (EFS) is 6 mos, for all responders + SD 8.7 mos versus 2.6 mos for others (P<0.0001). The median disease-free survival (DFS) for CR/CRi patients is 8.5 mos. The median time on study is 4.4 mos, and pts received median 4 cycles (range, 1-16).
Cohort 2: 22 newly dx pts have been enrolled and baseline characteristics are summarized in Table 1A. Among 17 evaluable pts, 8 achieved CR/CRi (6/2) (47%) (Table 1B), 2 PR (12%), 2 HI (12%), and 4 SD for at least 6 cycles (24%). The median # of cycles to response was 2 (range, 2-15). The 4- and 8-week mortality were 5% (pt received AZA 1 day only) and 9%, respectively. With a median follow-up of 19 mos, the median OS for the whole cohort is 13.1 mos, for responders + SD 13.4 mos (70% 1-yr) and not reached for CR/CRi/PR (79% 1-yr). The median EFS is 9.6 mos, for all responders + SD 13.4 mos versus 2.1 mos for others (P<0.0001). The median DFS for CR/CRi patients is 16.6 mos. The median time on study is 6.0 months, and pts received median 5 cycles (range, 1-21).
Toxicity: Grade 3/4 and grade 2 immune-related adverse events (IRAE) were observed in 9 (24%) and 5 (11%) pts in Cohort 1, and 3 (14%) and 4 (18%) pts in Cohort 2, respectively. The most common ≥grade 2 IRAEs included skin rash (n=7; 12%; 4 pts with prior alloSCT), hepatitis (n=5; 9%; 1 pt with grade 2 liver GVHD), pneumonitis (n=5; 9%), hypothyroidism (n=4; 7%), arthralgia (n=2; 3%), pericarditis (n=2; 3%), hyperglycemia (n=2; 3%), nephritis (1; 2%), uveitis (n=1; 2%), mucositis (n=1; 2%, pt with oral GVHD), and fevers. Ten pts required systemic steroids, including 3 pts with GVHD exacerbation. One pt (Cohort 1) died from multi-organ failure with grade 3 pneumonitis/hepatitis in the setting of PD, and two pts (Cohort 2) were referred to hospice for PD with grade 3/4 hepatitis, respectively, 1 of which resolved with steroids; the other was untreated. The rest of pts responded to steroids. The median time to onset of toxicities was 2 cycles (range, 1-9 cycles).
Conclusion: AZA/Pembro combination is safe, feasible and well tolerated for both R/R and newly dx older AML pts. Clinical activity is particularly notable in newly dx older AML pts. IRAEs occur and can be managed with steroids and supportive care in the majority of pts. Biomarker-based correlative studies are ongoing to better define specific subset of patients that may benefit from this approach.
Gojo:Abbvie: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Amphivena: Research Funding; Amgen Inc: Consultancy, Honoraria, Research Funding; Juno: Research Funding; Merck: Research Funding. Webster:Pfizer: Consultancy; Amgen: Consultancy; Genentech: Research Funding. Varela:Alexion: Speakers Bureau; NexImmune: Equity Ownership, Membership on an entity's Board of Directors or advisory committees. DeZern:Astex Pharmaceuticals, Inc.: Consultancy; Celgene: Consultancy. Foster:Bellicum Pharmaceuticals, Inc: Research Funding; MacroGenics: Research Funding; Celgene: Research Funding; Daiichi Sankyo: Consultancy. Levis:Astellas: Consultancy, Research Funding; FUJIFILM: Consultancy, Research Funding; Menarini: Consultancy, Honoraria; Novartis: Consultancy, Research Funding; Agios: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Daiichi Sankyo Inc: Consultancy, Honoraria. Coombs:Abbvie: Consultancy; Covance: Consultancy; Pharmacyclics: Honoraria; Cowen & Co.: Consultancy; Medscape: Honoraria; Octopharma: Honoraria; H3 Biomedicine: Honoraria; Loxo: Honoraria; Dedham Group: Consultancy. Smith:Novartis: Consultancy; Agios: Consultancy; Pfizer: Consultancy; Jazz: Consultancy; Celgene: Consultancy. Vincent:Merck: Research Funding; Pharmacyclics: Research Funding. Serody:Merck: Research Funding; GlaxoSmithKline: Research Funding. Luznik:AbbVie: Consultancy; WindMiL Therapeutics: Patents & Royalties: Patent holder; Merck: Research Funding, Speakers Bureau; Genentech: Research Funding. Zeidner:Tolero: Honoraria, Research Funding; Pfizer: Honoraria; AsystBio Laboratories: Consultancy; Daiichi Sankyo: Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Agios: Honoraria; Merck: Research Funding; Takeda: Research Funding; AbbVie: Honoraria.
Pembroluzimab - a PD1 checkpoint inhibitor which is not approved in AML (off-label drug use)
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract