Introduction:
Management of breakthrough bleeding events in patients on emicizumab involves episodic treatment with the bypassing agents (BPA), activated prothrombin complex concentrate (aPCC) and recombinant activated FVII (rFVIIa). A concomitant drug reaction between emicizumab and aPCC resulting in thrombotic events was noted in the HAVEN clinical trials resulting in a black box warning recommending avoiding treatment with high and prolonged doses of aPCC in patients on emicizumab. There have been a few reports where hemophilia plasma was spiked with emicizumab and then various concentrations of BPA which have demonstrated a synergistic effect of emicizumab with aPCC and an additive effect with rFVIIa using thrombin generation assays (TGA), however studies using in vivo emicizumab blood samples have not been performed. In addition, spiking with very low concentrations of aPCC and rFVIIa have not been done. With this in mind, we elected to assess the effect of spiking various concentrations of BPA on plasma taken from patients on emicizumab on thrombin generation.
Methods
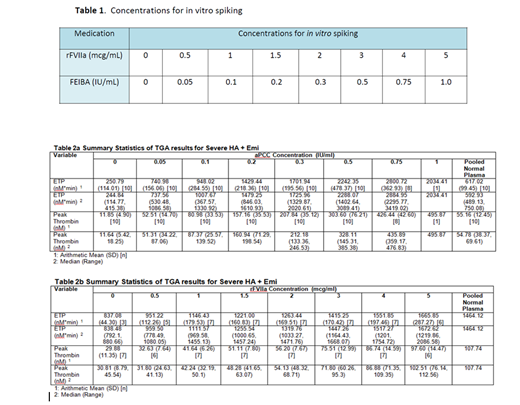
Patients with severe hemophilia A (HA) with inhibitors who are currently on emicizumab and who achieved steady state were recruited to participate. Blood samples were drawn in the non-bleeding state and with no recent use of BPA and TGA was performed using the calibrated automated thrombogram with platelet poor plasma (PPP) using the PPP reagent low for aPCC and with platelet rich plasma (PRP) using the PRP reagent for rFVIIa. Eight concentrations of aPCC and rFVIIa ranging from zero (baseline) to very low (sub-therapeutic) to therapeutic concentrations were spiked in vitro into patient samples. Table 1 describes the concentrations of the bypassing agents and descriptive statistics are provided for the different TGA parameters.
Results
Eleven patients with severe HA and inhibitors currently on emicizumab for at least 6 weeks were enrolled in the study. The TGA parameters assessed include lag time (min), ETP (nM*min), Peak thrombin (nM), time to peak (min), Vel index (nM/min) and start trail (min). The summary statistics are provided in tables 2a and 2b. The in vitro TGA spiking with aPCC demonstrated normalization of both the ETP and peak thrombin at a concentration of 0.05 IU/mL and 0.1 IU/mL comparable to a dose of ~5 IU/kg and ~10 IU/kg, respectively, however above this concentration, aPCC spiking demonstrated a very high ETP and peak thrombin (Table 2a). The in vitro TGA spiking with rFVIIa demonstrated normalization of ETP and peak thrombin at up to a concentration of 3 mcg/ml comparable to a dose of ~180 mcg/kg (Table 2b).
Discussion
Due to the known thrombotic complications when emicizumab is used in conjunction with aPCC, there has been a large-scale abandonment of the use of aPCC in patients on emicizumab leaving these inhibitor patients with only one BPA for management of bleeding and surgery which is not ideal. However, it is possible that aPCC can be used safely with emicizumab albeit with lower doses than may typically be used and below the doses in the prescribing information. While only clinical studies can answer this question, before this is attempted, a study of in vitro spiking of in vivo emicizumab samples should be performed. Thus, in this study we set out to determine what concentrations of BPA (in patients on emicizumab) could result in normalization of the TGA parameters ETP and peak thrombin. This, in turn, could suggest which doses of both rFVIIa and especially aPCC could be studied in a trial (or even used safely) when managing breakthrough bleeding or surgery in patients on emicizumab. The results demonstrate that at rather low concentrations of aPCC equating doses of ~5 and ~10 IU/kg, the ETP and peak thrombin reach normal levels, i.e. not excessive. We also note that most concentrations of rFVIIa result in normal ETP and peak thrombin as has been reported before. In conclusion, we have demonstrated that lower doses of aPCC could potentially be used safely and effectively in inhibitor patients on emicizumab. It would be important to test this hypothesis in a clinical study.
Young:Bioverativ/Sanofi: Consultancy, Honoraria; CSL Behring: Consultancy, Honoraria; Freeline: Consultancy, Honoraria; Genentech/Roche: Consultancy, Honoraria, Research Funding; Grifols: Consultancy, Honoraria; Kedrion: Consultancy, Honoraria; Novo Nordisk: Consultancy, Honoraria; Spark: Consultancy, Honoraria; Shire/Takeda: Consultancy, Honoraria; Uniqure: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.