Background
Venetoclax (Ven) is a selective, small molecule inhibitor of anti-apoptosis protein Bcl-2 that has antitumoral activity against multiple myeloma (MM), notably those with translocation t(11;14). Dexamethasone (d) has shown to enhance the expression of BCL-2 and pro-apoptotic protein Bim, shifting its binding towards Bcl-2 and sensitizing MM cells to Ven. This Phase 1/2 open-label trial is evaluating the safety and efficacy of the combination of Ven+d as a therapeutic approach to improve clinical outcomes in patients with t(11;14) Relapsed/Refractory (R/R) MM.
Methods
This ongoing study (NCT01794520) has 2 distinct phases. Phase 1 (P1) consisted of separate dose escalation, safety expansion, and Ven+d combination cohorts. The phase 2 (P2) portion is an expansion cohort of Ven+d to further evaluate its efficacy. The safety and efficacy of the Ven+d cohorts from P1 and P2 are reported here.
Eligible patients (pts) were adults with t(11;14) R/R MM ≥18 years of age. Key eligibility criteria for the Ven+d cohort in P1 included prior treatment with a proteasome inhibitor (PI) and an immunomodulatory drug (IMID), and an ECOG score ≤1. In P2, eligibility included an ECOG score ≤2, disease progression on or within 60 days from the last dose on previous treatment and having received at least two lines of therapy with a PI, IMID, daratumumab, and glucocorticoids. Additional inclusion criteria for both phases included measurable disease and adequate bone marrow function in all pts, and the presence of t(11;14) determined by fluorescence in-situ hybridization.
In both phases, pts received oral Ven 800 mg/day + oral d 40 mg (20 mg for pts ≥75 years of age) on days 1, 8, and 15 of each 21-day cycle. In P1, preliminary efficacy was assessed as the overall response rate (ORR), time to progression (TTP), progression free survival (PFS), and duration of response (DOR) of Ven+d based on the 2011 International Uniform Response Criteria for MM. Efficacy in P2 further evaluated ORR, very good partial response (VGPR) or better, PFS, DOR, TTP, and overall survival (OS) using 2016 IMWG response criteria.
Results
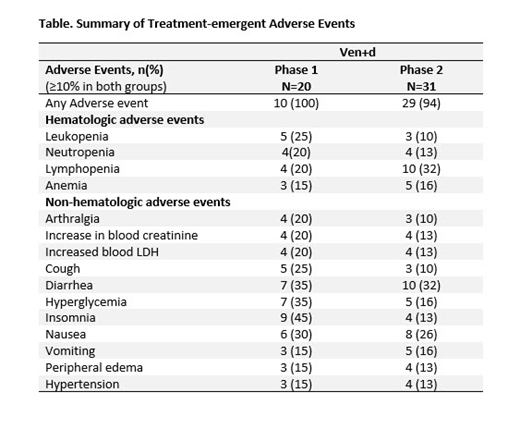
In P1, as of 15thApril, 2019, 20 pts were enrolled (17 [85%] male; median age, 63 years [range, 46-77]). The median number of prior therapies received was 2.5 (range: 1-7). Nineteen pts (95%) discontinued the study (18 due to progressive disease and 1 underwent transplant). The treatment-emergent adverse events (TEAEs) are summarized in the Table. Most frequent TEAEs were insomnia (45%), hypophosphatemia (40%), hyperglycemia (35%), diarrhea (35%), and thrombocytopenia (30%). Predominant Grade 3 or 4 TEAEs included neutropenia (21%), lymphopenia (29%), and hypophosphatemia (20%). The most common serious TEAE was tumor lysis syndrome (TLS) (10%). No deaths were reported in the treatment-emergent period. ORR was achieved by 13 (65%) and ≥VGPR was achieved by 6 (30%) pts respectively. Partial response (PR) was observed in 7 (35%) pts and stable disease (SD) in 4 (20%) pts. Median time to first response was 1.4 mos (95% CI: 0.7, 2.8). The DOR was 13.1 mos (95% CI: 10.9, 21.9). Median PFS and TTP were both 12.4 mos (95% CI 3.6, 20.9).
In P2, as of March 18, 2019, 31 pts were enrolled (18 [58%] male; median age, 65 years [range, 48-80]). The median number of prior therapies received were 5 (range: 1-9). Eight (26%) pts discontinued the study (6 deaths, 1 withdrew consent, 1 due to physician's decision). The most frequent TEAEs reported were diarrhea (32%), nausea (26%) and lymphopenia (32%). The most frequent Grade 3 or 4 TEAEs were lymphopenia (32%), thrombocytopenia (11%), and hypertension (10%). Most common serious TEAE was sepsis (10%). Six (19%) deaths were reported (5 due to progressive disease and 1 due to adverse event). In the intention to treat population, the median TTR was 0.7 mos (range: 0.6-1.5). ORR was achieved by 14 (45%) and >VGPR was observed in 8 (26%) pts. PR was observed in 6 (13%) pts and SD in 8 (26%) pts. Median PFS, DOR, and OS were not reached at the time of data analysis. The 9-mo estimates for PFS, DOR, and OS were 57% (95% CI: 19% 82%), 66% (95% CI: 16%, 91%), and 71% (95% CI: 44%, 87%) respectively.
Conclusion
In both phases of this study, Ven+d was efficacious and demonstrated tolerable safety in pts with t(11;14) R/R MM. These results support further investigation of Ven combinations in this pt population. This combination is also being investigated in the ongoing Phase 3 trial M13-494 (CANOVA) in t(11;14) positive R/R MM.
Kaufman:Takeda: Consultancy; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Bristol-Myers Squibb: Consultancy; Incyte: Consultancy; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; AbbVie: Consultancy; Winship Cancer Institute of Emory University: Employment; Amgen: Consultancy; TG Therapeutics: Consultancy. Gasparetto:Celgene: Consultancy, Honoraria, Other: Travel, accommodations, or other expenses paid or reimbursed ; Janssen: Consultancy, Honoraria, Other: Travel, accommodations, or other expenses paid or reimbursed ; BMS: Consultancy, Honoraria, Other: Travel, accommodations, or other expenses paid or reimbursed . Schjesvold:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; SkyliteDX: Honoraria. Moreau:Takeda: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Celgene: Consultancy, Honoraria. Facon:Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Boise:Genentech Inc.: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Research Funding. Alzate:AbbVie Inc: Employment, Other: Stock/stock options. Macartney:AbbVie Inc: Employment, Other: Stock/stock options. Pesko:AbbVie: Employment, Other: Stock/stock options. Salem:AbbVie: Employment, Other: Stock/stock options. Ross:AbbVie: Employment, Other: Stock/stock options. Hong:Genentech Inc.: Employment, Equity Ownership; Roche: Equity Ownership. Maciag:AbbVie: Employment, Other: Stock/stock options. Pauff:AbbVie Inc: Employment, Other: may own stock or stock options. Kumar:Takeda: Research Funding; Celgene: Consultancy, Research Funding; Janssen: Consultancy, Research Funding.
Venetoclax is a BCL-2 inhibitor that is FDA-approved in some indications. This presentation will focus on venetoclax for treatment in multiple myeloma, which is not an approved indication.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract