Background: Sickle cell disease (SCD) comprises a group of genetic blood disorders caused by a single missense mutation (Glu6Val) in the β-globin gene. In early childhood, SCD progresses into a systemic disease resulting in complications that include vaso-occlusion, multi-organ damage, and early death. P-selectin, an adhesion molecule expressed on activated vascular endothelial cells and platelets, contributes to the cell-to-cell and cell-to-endothelium interactions that are involved in the pathogenesis of vaso-occlusive crisis (VOC) in SCD. Crizanlizumab is an investigational, humanized, anti-P-selectin monoclonal antibody under evaluation for the prevention of VOCs in patients with SCD. In the Phase II SUSTAIN study, crizanlizumab 5.0 mg/kg significantly reduced the median annual rate of VOCs compared with placebo (P=0.010). The purpose of the randomized, double-blind, Phase III placebo-controlled STAND study is to compare the efficacy and safety of two doses of crizanlizumab (5.0 and 7.5 mg/kg) versus placebo in adolescent and adult patients with SCD and a history of VOCs leading to a healthcare visit.
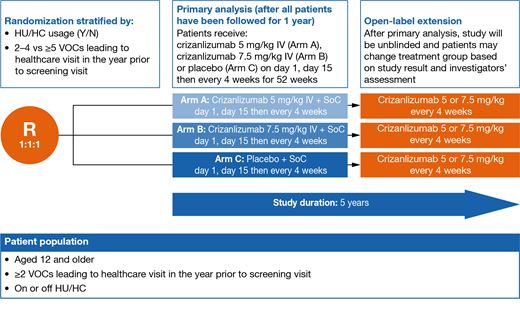
Methods: The STAND study aims to randomize 240 patients with SCD (all SCD genotypes eligible) aged ≥12 years (including at least 48 adolescents), who experienced ≥2 VOCs leading to a healthcare visit in the 12 months prior to screening, and who are not planning to initiate hydroxyurea (HU)/hydroxycarbamide (HC) or erythropoietin-stimulating agents (ESAs) or L-glutamine during the trial. Patients who have been taking HU/HC, ESAs or L-glutamine for ≥6 months and plan to continue the same dose at least until they reach 1 year of investigational treatment will be permitted. Exclusion criteria include: history of stem cell transplant; receipt of blood products within 30 days of the first dose; participation in a chronic exchange or transfusion program; and receipt of therapeutic anticoagulation or antiplatelet therapy within the 10 days prior to the first dose.
Patients in the study will be randomized into 1 of 3 treatment arms: crizanlizumab 5.0 mg/kg, 7.5 mg/kg, or placebo administered intravenously over a period of 30 minutes on week 1 day 1, week 3 day 1, and day 1 of every 4-week cycle thereafter.
Primary analysis cut-off date will occur once all patients have reached 1 year of treatment or discontinued within year 1. An open-label extension will offer the possibility to switch treatment (Figure).
The primary endpoint is the annualized rate of VOCs leading to a healthcare visit in each treatment group over the first year of treatment. The key secondary endpoint is the annualized rate of all VOCs (leading to a healthcare visit or treated at home) over the first year post randomization. Other objectives include the rate of patients free from VOCs leading to a healthcare visit, time to first and second VOC leading to a healthcare visit, number of days with VOCs leading to a healthcare visit, healthcare resource utilization, SCD-related renal damage, pharmacokinetics and pharmacodynamics (P-selectin inhibition), immunogenicity, biomarkers and quality of life.
The primary efficacy endpoint will be analyzed based on the data from the full analysis set comprising all randomized patients. A negative binomial regression model with treatment and randomization stratification factors as covariates will be used for analysis, with the logarithm of observation time as offset. The estimates of annualized VOC rates between treatment groups and their 95% confidence intervals will be provided [NCT03814746, EudraCT 2017-001746-10].
Conclusion: This study is designed to confirm the efficacy and safety of crizanlizumab 5 mg/kg and assess the safety and efficacy of a higher dose (7.5 mg/kg) in patients with SCD and history of VOCs.
Abboud:GBT: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Other: Travel support; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; CRSPR Therapeutics: Membership on an entity's Board of Directors or advisory committees; Modus: Research Funding; Eli Lilly: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Novo Nordisk: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Howard:Resonance Health: Other: Travel grant; Global Blood Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel grant; Imara: Consultancy, Other: Travel grant. Cançado:Novartis: Membership on an entity's Board of Directors or advisory committees. Smith:Novartis: Consultancy, Honoraria. Espurz:Novartis Pharma AG: Employment. Weill:Novartis Pharma AG: Employment. de Montalembert:bluebird bio, Inc: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AddMedica: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.