In this issue of Blood, Morales-Hernández et al describe an essential role for G protein–coupled receptor–associated sorting proteins (GPRASP) in regulating hematopoietic stem cell (HSC) survival, proliferation, and in vivo engraftment capacity, findings with potentially significant implications for the current practice of hematopoietic cell transplantation.1 Hematopoietic cell transplantation provides curative therapy for thousands of patients with hematologic malignancies, bone marrow (BM) failure, and other life-threatening hematologic disorders.2 However, many patients who meet criteria to benefit from hematopoietic cell transplantation lack an HLA-matched related or unrelated donor,3 and alternative donor transplantation, utilizing haploidentical related donors or cord blood, presents challenges related to relapse rate and graft failure risk, respectively.4,5 Exciting advances in HSC gene editing and gene transfer have reignited clinical gene therapy trials for hematologic and immune deficiency diseases, but ex vivo genetic manipulation of HSCs can diminish HSC engraftment capacity in vivo.6 The development of techniques to enhance the repopulating capacity of donor HSCs could have broad impact in hematopoietic cell transplantation and gene therapy. However, the development of translatable methods to enhance human HSC engraftment has been impeded by a lack of understanding of the mechanisms that control HSC engraftment following transplantation. In this issue of Blood, Morales-Hernández et al describe the fascinating role of GPRASPs in regulating HSC repopulating capacity following transplantation.
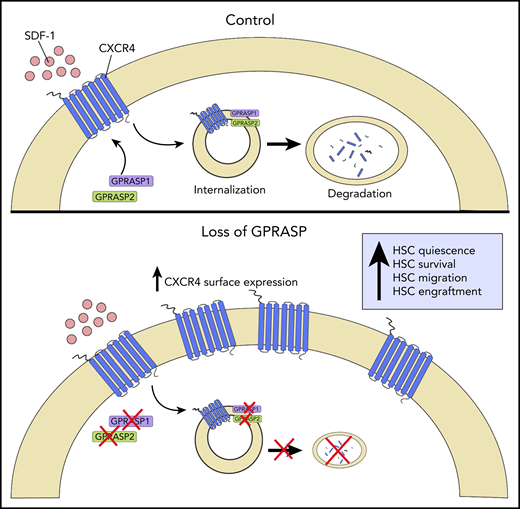
Schematic representation of GPRASP control of CXCR4-mediated HSC functions. In homeostasis (Control), GPRASP1 and GPRASP2 regulate CXCR endocytic trafficking and degradation. In the absence of GPRASP1 or GPRASP2, CXCR4 accumulates in HSCs, promoting HSC quiescence, survival, migration, and engraftment capacity. Illustration by Christina M. Termini, UCLA.
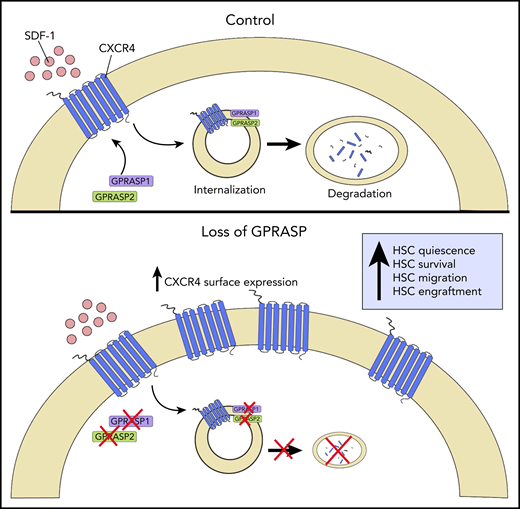
Schematic representation of GPRASP control of CXCR4-mediated HSC functions. In homeostasis (Control), GPRASP1 and GPRASP2 regulate CXCR endocytic trafficking and degradation. In the absence of GPRASP1 or GPRASP2, CXCR4 accumulates in HSCs, promoting HSC quiescence, survival, migration, and engraftment capacity. Illustration by Christina M. Termini, UCLA.
The authors discovered that GPRASP2 is expressed by murine HSCs and that short hairpin RNA (shRNA)-mediated silencing of Gprasp2 increased murine HSC repopulating capacity.7 In the current study, the authors show that murine HSCs also differentially express Gprasp1, which shares homology with Gprasp2. Interestingly, congenic mice competitively transplanted with lineage negative sca-1+ckit+ (LSK) hematopoietic stem/progenitor cells (HSPCs) treated with viral shRNA-Gprasp2 or shRNA-Gprasp1 displayed two- to threefold increased donor hematopoietic cell engraftment through 20 weeks posttransplant compared with recipients of control shRNA-treated LSK cells. These results suggest that GPRASP1 and GPRASP2 proteins are negative regulators of HSC engraftment following transplantation.
The authors next analyzed the in vivo engraftment capacity of BM HSCs from Gprasp1−/− mice and Gprasp2−/− mice and discovered that BM cells from neither mouse displayed a difference in engraftment capacity in vivo compared with wild-type BM cells. Compensatory upregulation of another GPRASP family member, Bhlhb9, was identified in HSPCs from Gprasp1−/− and Gprasp2−/− mice. shRNA-mediated silencing of Bhlhb9 in BM LSK cells from Gprasp1−/− or Gprasp2−/− mice resulted in increased early engraftment of transplanted LSK cells, consistent with that observed with shRNA targeting Gprasp1 and Gprasp2, although mice transplanted Bhlhb9-deficient HSCs demonstrated decreased donor engraftment at or beyond 12 weeks posttransplant compared with controls. Therefore, the authors identified a third GPRASP protein, BHLHB9, that also regulates early HSC engraftment capacity, albeit with distinct effects on long-term HSC engraftment compared with GPRASP1 or GPRASP2.
GPRASP proteins are known to regulate the postendocytic trafficking of G protein–coupled receptor (GPCRs) via binding to their C-terminal and GASP domains.8 The authors discovered that GPRASP1 and 2 proteins physically colocalize with CXC chemokine receptor 4 (CXCR4), a GPCR that regulates HSC function via binding to CXCL12.9 Furthermore, silencing of Gprasp1 or Gprasp2 in HSPCs caused the accumulation of CXCR4 first in the cytoplasm and then at the cell surface, due to delayed CXCR4 degradation.
Gprasp1- and Gprasp2-deficient HSPCs displayed decreased apoptosis and increased quiescence in short-term culture compared with control HSPCs. Gprasp1- and Gprasp2-deficient long-term HSCs also displayed increased survival and quiescence in vivo following transplantation into congenic mice, in association with elevated levels of CXCR4.
In keeping with the effects on CXCR4 accumulation, Gprasp1- and Gprasp2-deficient HSPCs displayed increased transwell migration to stromal cell–derived factor 1 in vitro and augmented homing to the BM within 3 hours posttransplant. Gprasp1- and Gprasp2-deficient HSCs also demonstrated superior niche retention in response to cyclophosphamide/granulocyte colony-stimulating factor mobilization in mice compared with control HSCs. Taken together, these results suggested that GPRASP1 and GPRASP2 regulate HSPC homing and retention in the niche via control of CXCR4 levels.
Importantly, the authors further demonstrated that silencing of Gprasp1 or Gprasp2 had no effect on HSPC survival, quiescence, or migration in vitro in CXCR4-deficient HSPCs. Whereas silencing of Gprasp1 or Gprasp2 in CXCR4-expressing HSCs increased in vivo engraftment in recipient mice, the authors observed no effects on the in vivo engraftment capacity of CXCR4-deficient HSCs. Taken together, these results confirm that GPRASP1- and GPRASP2-mediated control of HSPC survival, quiescence, migration, and in vivo engraftment capacity is dependent on the presence of CXCR (see figure).
This study highlights the important role for GPRASP1 and GPRASP2 proteins in regulating HSPC function via CXCR4. The results also raise exciting fundamental questions and possibilities regarding the broader role of GPRASP proteins in regulating other GPCRs expressed by HSCs.10 At the same time, GPRASP1 and GPRASP2 represent attractive new molecular targets for pharmacologic or biologic interventions to enhance human HSC engraftment and mobilization in patients.
Conflict-of-interest disclosure: J.P.C. declares no competing financial interests.