TO THE EDITOR:
Coronavirus disease 2019 (COVID-19) has emerged as a global pandemic associated with a strikingly high rate of morbidity and mortality.1,2 There is growing evidence that a pathophysiologic component of severe COVID-19 disease may be related to a provoked procoagulant state.3-6 High rates of thromboembolic complications of COVID-19 infection have been reported,7-11 and autopsy studies have identified evidence of macro- and microembolism in COVID-19–infected patients.12,13 Further, perturbations of coagulation markers, most notably dramatic elevations in D-dimer levels, have been noted among COVID-19 patients and have been associated with increased mortality.3,14
Empiric therapeutic anticoagulation (AC) is now being used in clinical practice at many centers and will be evaluated in randomized clinical trials; however, despite the rationale for therapeutic AC, the efficacy of such an approach remains largely untested. We sought to provide evidence for or against the use of therapeutic AC among these patients. To this end, we performed a retrospective analysis of patients with confirmed COVID-19, comparing outcomes among those who were and were not receiving AC for unrelated indications at the time of COVID-19 diagnosis. Our hypothesis was that AC prior to (and during the earliest stages of) COVID-19 infection would be protective for COVID-19–related outcomes.
We retrospectively reviewed all patients with laboratory-confirmed COVID-19 diagnosed across a large New York City health system between 1 March 2020 and 1 April 2020. Confirmed COVID-19 was defined by a positive result on a reverse transcriptase polymerase chain reaction severe acute respiratory syndrome coronavirus 2 assay. Hospitalized and ambulatory patients were included in the analysis. The primary outcome was all-cause mortality. Relevant secondary outcomes included hospitalization, need for invasive mechanical ventilation, new initiation of renal replacement therapy, imaging-confirmed thrombosis, and major (World Health Organization grade ≥3) bleeding.15 This study was approved by the Program for the Protection of Human Subjects of the Icahn School of Medicine at Mount Sinai and conducted in accordance with the Declaration of Helsinki.
To adjusted for bias due to nonrandom allocation of potential covariates among COVID-19 patients, we applied propensity score-matching methods.16 Propensity scores were calculated using a logistic regression model, adjusting for the following covariates: age, sex, race, Charlson Comorbidity Index and obesity. A 1:3 match was performed using Greedy matching techniques.16 Two separate analyses, time-to-event analysis and event analysis, were performed for the outcomes all-cause mortality and mechanical ventilation. Event analysis only was performed for the outcome hospitalization. The results of time-to-event analyses were expressed as Kaplan-Meier curves, with significance indicated using a log-rank P value. Cox proportional-hazard models of all-cause mortality, mechanical ventilation, and hospitalization among the different propensity-matched comparisons groups with robust sandwich variance estimates of standard errors were performed, and the results are expressed as hazard ratios (HRs) with 95% confidence interval (CI).
We identified 4343 consecutive patients with laboratory-confirmed COVID-19 between 1 March 2020 and 1 April 2020. A total of 571 patients were excluded because they were younger than 18 years of age (n = 55) or had insufficient clinical documentation because they had been diagnosed at a rapid testing center (n = 516), resulting in a final study population of 3772 patients. There were 241 patients receiving AC, 672 patients receiving antiplatelet therapy, and 2859 patients not receiving AC or antiplatelet therapy at the time of COVID-19 diagnosis (Table 1). All patients in the AC group were continued on AC following COVID-19 diagnosis. Overall, 53.8% of patients required hospitalization, 13.8% required mechanical ventilation, and 15.0% died (Table 1).
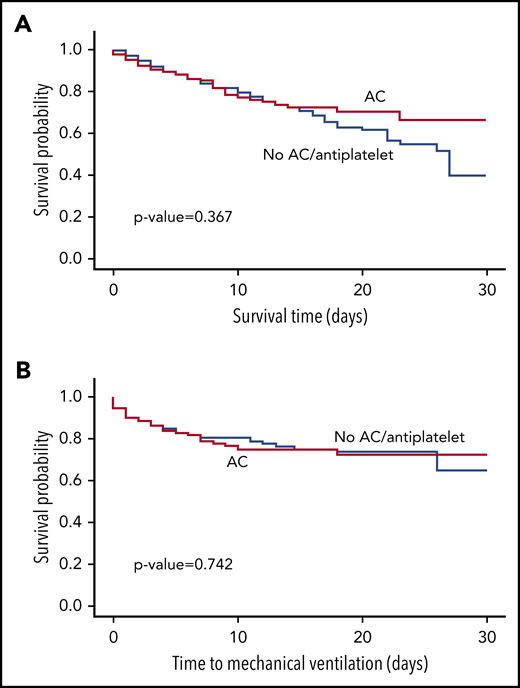
We first performed a propensity-matched analysis of patients who were on AC prior to COVID-19 infection compared with those who were not on AC or antiplatelet therapy. Propensity matching yielded 139 patients who received AC and 417 patients who did not receive treatment, with balanced variables between the groups (supplemental Table 1, available on the Blood Web site). There was no statistically significant difference in survival (P = .367; Figure 1A) or time-to-mechanical ventilation (P = .742; Figure 1B) between the 2 groups. The HRs for all-cause mortality, mechanical ventilation, and hospital admission in the AC vs no-AC/antiplatelet groups were 1.208 (95% CI, 0.750-1.946), 0.905 (95% CI, 0.571-1.435), and 1.027 (95% CI: 0.654-1.612), respectively. We performed the same analysis but compared patients receiving antiplatelet therapy vs patients not receiving antiplatelet therapy or AC prior to COVID-19 infection (supplemental Table 2). Again, there was no statistically significant difference in survival (P = .997) or time-to-mechanical ventilation (P = .256) between the 2 groups (supplemental Figure 1). The HRs for all-cause mortality, mechanical ventilation, and hospital admission in the antiplatelet therapy group vs the no AC/antiplatelet group were 1.029 (95% CI, 0.723-1.466), 1.239 (95% CI, 0.807-1.901), and 0.989 (95% CI, 0.755-1.296), respectively.
Survival and time-to-mechanical ventilation analysis in the propensity-matched AC cohort compared with no-AC/antiplatelet cohort. Propensity matching yielded 139 patients who received AC and 417 patients who did not receive AC or antiplatelet therapy; they were compared using Kaplan-Meier survival analysis. (A) There was no statistically significant difference in survival between those who received AC and the propensity-matched control group (P = .367). (B) There was no statistically significant difference in time-to-mechanical ventilation between the 2 groups (P = .742).
Survival and time-to-mechanical ventilation analysis in the propensity-matched AC cohort compared with no-AC/antiplatelet cohort. Propensity matching yielded 139 patients who received AC and 417 patients who did not receive AC or antiplatelet therapy; they were compared using Kaplan-Meier survival analysis. (A) There was no statistically significant difference in survival between those who received AC and the propensity-matched control group (P = .367). (B) There was no statistically significant difference in time-to-mechanical ventilation between the 2 groups (P = .742).
Given the absence of effective COVID-19–directed therapies to date, there remains great interest in unconventional or repurposed approaches. Therapeutic AC has been used in prior pandemics of respiratory viruses, such as H1N1 influenza.17,18 The ongoing uncertainty regarding the role of a procoagulant state in the pathophysiology of severe COVID-19 has led some to use AC as a therapeutic modality; however, rigorous data are lacking. In a cohort of 449 COVID-19 patients from Wuhan, China, prophylactic heparin was used in 99 patients and was associated with an improvement in mortality in a specific subgroup (those with a sepsis-induced coagulopathy score ≥4). However, the rate of prophylactic AC was low, and the reasoning behind its implementation patterns was not provided.19
The findings of the present study are limited to an assessment of the impact of prediagnosis AC among patients with COVID-19 infection. Our study does not exclude the possibility that therapeutic AC may have a role among some patients with severe COVID-19 infection, particularly those who are critically ill. However, evaluation of this therapeutic strategy in a prospective randomized control trial is urgently needed to fully assess its efficacy and safety. Currently, the routine use of empiric therapeutic AC among patients with COVID-19 is not recommended by the American Society of Hematology.20 Although the findings described herein support this position, they do not rule out the possibility that, among some subgroups of COVID-19 patients, therapeutic AC following diagnosis may be of utility. Future research endeavors should be aimed at identifying these groups of patients.
There are several important limitations to the current study, including its retrospective nature. We examined all patients, both ambulatory and hospitalized; however, there are many patients carrying COVID-19 who were never tested and, therefore, were not evaluated in this analysis. Although we included a number of factors in our propensity score matching, there are factors that were not included that could impact mortality, need for mechanical ventilation, or hospitalization. We also did not examine the influence of interventions after patients were hospitalized, which could conceivably impact our outcomes (particularly with respect to in-hospital prophylactic AC). However, prediagnosis AC was not associated with a decreased rate of hospitalization, suggesting that AC does not protect against development of severe COVID-19 disease. It is also possible that, if thrombotic complications are more a feature of later-stage disease, studying exposure to AC early in the disease course may fail to detect latent benefit. Strengths of our study include a very large consecutive inclusive cohort during the peak of the COVID-19 pandemic, with clinically relevant exposures and outcomes, as well as a rigorous statistical analysis.
Our results suggest that AC alone is unlikely to be protective for COVID-19–related morbidity and mortality. Nevertheless, it bears reemphasizing that further studies, particularly prospective controlled trials, are needed to validate these finding and identify appropriate patients for whom therapeutic AC may be beneficial.
Data sharing requests should be sent to Leonard Naymagon (leonard.naymagon@mountsinai.org).
The online version of this article contains a data supplement.
Acknowledgments
The authors acknowledge the Anticoagulation and COVID-19 Working Group at Mount Sinai for their input on thrombosis and COVID-19. They also acknowledge Teja Ganta for assisting with data collection.
Authorship
Contribution: D.T. and L.N. designed the study, analyzed the data, and wrote the manuscript; D.T., M.v.G., M.A., S.T., A.K., S.V., I.M., Q.Q., S.D., T.J., S. Bhalla, S. Berwick, J.F., and L.N. collected clinical data; M.v.G. and M.A. performed statistical analyses; J.M., K.T., C.C., A.D., and W.K.O. provided critical input and analysis; and all authors wrote and edited the manuscript.
Conflict-of-interest disclosure: J.M. has received research funding from CTI Biopharma. A.D. has received research funding from Pfizer/Bristol-Myers Squibb, served on the scientific advisory board for Bristol-Myers Squibb, and received an honorarium from Johnson & Johnson. W.K.O. has acted as a consultant for Astellas, AstraZeneca, Bayer, Janssen, Sanofi, Sema4, and TeneoBio. The remaining authors declare no competing financial interests.
Correspondence: Leonard Naymagon, Division of Hematology and Medical Oncology, Department of Medicine, Icahn School of Medicine at Mount Sinai, One Gustave L. Levy Pl, Box 1079, New York, NY 10029; e-mail: leonard.naymagon@mountsinai.org.