Key Points
Adding sirolimus to standard GVHD prophylaxis reduces acute GVHD after nonmyeloablative HLA antigen–mismatched donor transplantation.
Compared with historical control subjects, the reduced incidence of acute GVHD translates into a better overall survival.
Abstract
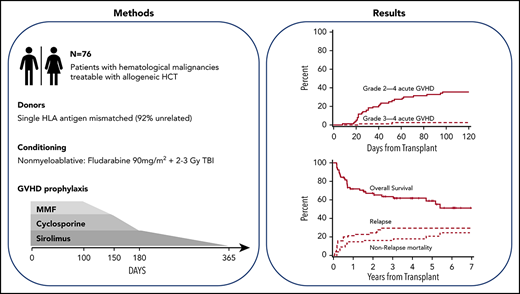
This trial aimed to evaluate the efficacy of sirolimus in addition to cyclosporine (CSP) and mycophenolate mofetil (MMF) for graft-versus-host disease (GVHD) prophylaxis after nonmyeloablative conditioning for HLA class I or II mismatched hematopoietic cell transplantation (HCT). Eligible patients had hematologic malignancies treatable by allogeneic HCT. Conditioning consisted of fludarabine (90 mg/m2) and 2 to 3 Gy total body irradiation. GVHD prophylaxis comprised cyclosporine, mycophenolate mofetil, and sirolimus. The primary objective was to determine whether the cumulative incidence of grade 2 to 4 acute GVHD could be reduced to <70% in HLA class I or II mismatched HCT. The study was closed on December 20, 2018. Seventy-seven participants were recruited between April 14, 2011, and December 12, 2018, of whom 76 completed the study intervention. Median follow-up was 47 months (range, 4-94 months). The cumulative incidence of grade 2 to 4 acute GVHD at day 100 was 36% (95% confidence interval [CI], 25-46), meeting the primary end point. The cumulative incidence of nonrelapse morality, relapse/progression, and overall survival was 18% (95% CI, 9-27), 30% (interquartile range, 19-40), and 62% (95% CI, 50-73) after 4 years. In conclusion, the addition of sirolimus to cyclosporine and mycophenolate mofetil resulted in a lower incidence of acute GVHD, thus translating into superior overall survival compared with historical results. This trial was registered at www.clinicaltrials.gov as #NCT01251575.
Introduction
With the development of nonmyeloablative regimens, allogeneic hematopoietic cell transplantation (HCT) with related or unrelated donors has become a viable treatment option for older or medically unfit patients with hematologic malignancies.1-3 A fully HLA-matched donor is considered ideal for the best possible outcome after allogeneic HCT.4 However, depending on the recipient’s ethnicity, fully HLA-matched donors can be identified for only 16% to 75% of patients.5 In an effort to extend this treatment option to patients who do not have HLA-matched donors available, we completed a phase 1/2 trial that included 59 patients who received unmodified peripheral blood stem cells (PBSCs) from a related or unrelated donor who was mismatched for either one HLA class I antigen with or without an additional mismatch for a HLA class I allele or mismatched for two HLA class I alleles.6 Graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporine and mycophenolate mofetil for 365 and 156 days, respectively. All but 2 patients achieved sustained engraftment, and the relapse incidence was encouragingly low at 26% at 2 years. However, the cumulative incidence of grade 2 to 4 acute GVHD and grade 3 to 4 acute GVHD was high at 69% and 26%, respectively. This resulted in a high cumulative incidence of nonrelapse mortality (NRM) of 47% by 2 years after transplantation. The overall survival was 29% at 2 years after transplantation.
The current multicenter phase 2 clinical trial was designed to improve both acute GVHD prevention and survival after nonmyeloablative conditioning HCT with HLA-mismatched donors by adding the mammalian target of rapamycin inhibitor, sirolimus, to standard therapy with cyclosporine and mycophenolate mofetil.7 The range of HLA mismatches allowed was expanded to include recipients of both HLA class I or class II antigen–mismatched donors with or without additional HLA allele-level mismatches.
Methods
Study design and participants
The phase 2 trial included 4 HCT centers (supplemental Table 1, available on the Blood Web site). The Fred Hutchinson Cancer Research Center acted as the coordinating center. The study was approved by the institutional review boards at the Fred Hutchinson Cancer Research Center and each of the collaborating centers. All patients signed institutional review board–approved consent forms. This trial was registered and is available for viewing at www.clinicaltrials.gov as #NCT01251575.
Included in this study were patients with advanced hematologic malignancies (Table 1) treatable by allogeneic HCT but not eligible for myeloablative conditioning, and having an HLA class I or II mismatched related or unrelated donor available. Three categories of HLA mismatches between the recipient and donor were allowed: (1) an HLA antigen mismatch for any single class I locus (HLA-A, HLA-B, or HLA-C) with or without an additional single class I mismatch at the allele level; (2) allele level mismatches for any two HLA class I loci; and (3) single antigen or allele level mismatches for HLA-DRB1 and/or HLA-DQB1 class II loci. Unmodified PBSCs from related or unrelated donors were the sole graft source. Eligibility criteria and patient evaluations are described in the supplemental Methods.
The protocol was opened on 14 April 2011, and closed on 12 December 2018, after accruing 77 patients. Results were analyzed as of 11 June 2019. The trial was stopped just short of meeting its accrual goal of 80 patients due to a new competing trial.
Procedures
Patients were conditioned for HCT with fludarabine (30 mg/m2 per day) on days 4, 3, and 2 before receiving 2 or 3 Gy total body irradiation (TBI) (n = 30 [39%]) on the day of HCT (day 0). Patients were treated with 3 Gy TBI if they had the following: myelodysplastic syndrome, myeloproliferative disease, or chronic myelocytic leukemia; patients who had no previous myelosuppressive chemotherapy within 3 to 6 months before entering the trial; and patients who had a previous allogeneic HCT or a prior syngeneic transplantation without subsequent myelosuppressive chemotherapy. PBSC grafts were collected from related or unrelated donors according to National Marrow Donor Program standards after 5 days of subcutaneously administered granulocyte colony-stimulating factor at a dose of ∼10 µg/kg. GVHD prophylaxis comprised sirolimus, cyclosporine, and mycophenolate mofetil. Cyclosporine was started at 5 mg/kg orally twice daily on day −3 and continued to day 150 and then tapered off by day 180. Cyclosporine trough levels were targeted at 400 ng/mL for the first 28 days and thereafter between 150 and 350 ng/mL until taper. Sirolimus was started orally at 2 mg once daily on day −3 and adjusted to maintain trough levels between 3 and 12 ng/mL through day 180, followed by taper through day 365. Mycophenolate mofetil was given orally at a dose of 15 mg/kg three times daily from day 0 to 30, then twice daily to day 100, and tapered to day 150. Supportive care, antibiotic prophylaxis, and detection and treatment of cytomegalovirus reactivation were conducted according to local institutional guidelines. Diagnosis, clinical grading, and treatment of acute and chronic GVHD were performed according to established criteria.10,11 Full donor chimerism was defined as >95% donor CD3+ T cells, and graft rejection was defined as the inability to detect at least 5% donor CD3+ T cells in peripheral blood. Detectable CD3+ T-cell donor chimerism in blood or bone marrow was considered evidence of engraftment.
Outcomes
The primary objective was to determine whether the cumulative incidence of grade 2 to 4 acute GVHD could be reduced to less than the historical rate of 70% with the combination of cyclosporine, sirolimus, and mycophenolate mofetil in HLA class I or HLA class II mismatched related or unrelated HCT using nonmyeloablative conditioning. The evaluation was performed separately among patients with HLA class I and HLA class II mismatches. Secondary objectives were time from transplantation to death without relapse or progression (NRM) before day 100 and the cumulative incidence of grade 3 to 4 acute GVHD. Exploratory outcomes were donor chimerism levels, engraftment, cumulative incidence of overall survival (time from transplantation to death from any cause), progression-free survival (time from transplantation to relapse/progression of the malignant disease or death from any cause), relapse (time from transplantation to relapse/progression of the malignant disease), chronic GVHD, infections, and Common Terminology Criteria for Adverse Events (version 4.0) of grade 3, 4, and 5 adverse events.
Statistics
The accrual target of 80 patients was anticipated to include ∼55 HLA class I mismatches and 25 HLA class II mismatches. For HLA class I mismatches, this provided 81% power to detect a 15% reduction in the 70% historical rate of grade 2 to 4 acute GVHD at the one-sided 0.10 level of significance, and 81% power to detect a 10% reduction in the 25% historical rate of grade 3 to 4 GVHD at the one-sided 0.15 level of significance. For HLA class II mismatches, there was 79% power to detect a 20% reduction in the historical rate at the one-sided 0.10 level of significance, and 68% power to detect a 10% reduction in the historical rate at the one-sided 0.20 level of significance. Seventy-seven patients were enrolled. The transplant procedure was aborted for 1 patient before receiving donor cells because of Merkel cell carcinoma; this patient is excluded from this report as being unevaluable for the primary end point, leaving 51 HLA class I mismatches and 25 class II mismatches.
The Kaplan-Meier method was used to estimate overall survival and progression-free survival; end points subject to competing risks were estimated by using cumulative incidence methods, with death as a competing risk for all end points and relapse as an competing risk for NRM.12 Cox regression was used for all time-to-event end points, with competing risks analysis based on event-specific hazard ratios. P values for time-to-event end points refer to hazard ratio analyses over the period of follow-up. Comparative analyses of toxicity and GVHD organ stage were conducted with a χ2 test. The Wilcoxon rank sum test was used for comparing peripheral blood counts and donor chimerism. P values are two-sided, and adjustments for multiple comparisons were not performed. Statistical analyses were performed by using SAS version 8 (SAS Institute, Inc., Cary, NC).
Results
Seventy-six patients were evaluable for outcome analyses. The median follow-up of surviving patients was 47 months (range, 4-94 months). Patient characteristics are summarized in Table 1. As part of their conditioning, 30 patients (39%) were treated with 2 Gy TBI and 46 (61%) with 3 Gy TBI.
Fifty (66%) patients had single HLA class I antigen mismatches, and 25 (33%) patients were mismatched for HLA class II. Unmodified PBSC grafts were obtained primarily from unrelated donors (n = 70 [92%]).
Sustained engraftment was observed in all but one (1%) patient who had myeloproliferative disease and rejected after 9 months. The patient had a single HLA-C antigen mismatch in both graft-versus-host and host-versus-graft directions. Among surviving and evaluated patients, median donor T-cell chimerism was 93% (interquartile range [IQR], 76-99) at day 28 and 97% (IQR, 85-100) at day 84. Full donor T-cell chimerism was achieved in 48% (95% confidence interval [CI], 36-61) and 63% (95% CI, 49-76) of patients alive and evaluated at days 28 and 84, respectively.
The median absolute neutrophil count nadir and the median period of neutrophils <500 cells/µL were 110 cells/µL (IQR, 30-300 cells/µL) and 14 days (IQR, 10-17 days), respectively. The median platelet count nadir was 14 000 cells/µL (IQR, 9000-25 000 cells/µL) and the median period of <20 000 platelets/µL was 2 days (IQR, 0-5 days).
Two patients (3%) were treated with donor leukocyte infusion, 1 for relapse of acute myeloid leukemia and 1 to correct low donor chimerism.
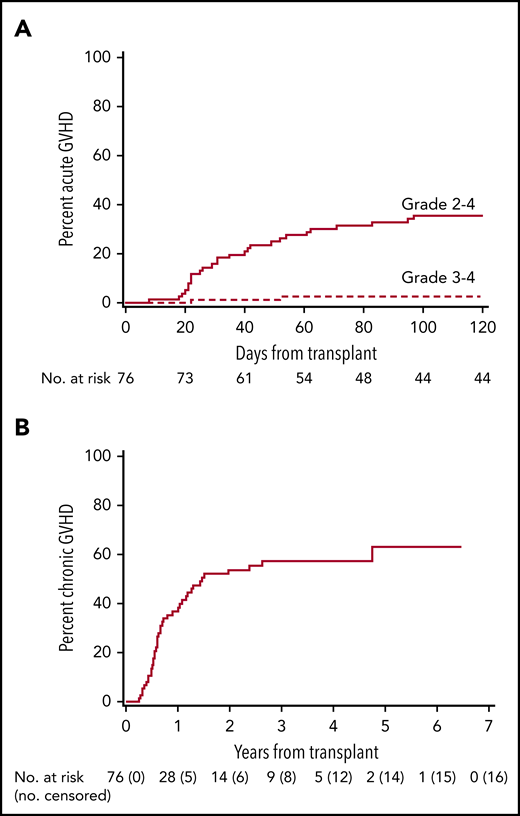
The cumulative incidence of grade 2 to 4 acute GVHD at day 100 was 36% (95% CI, 25-46) in all patients (Figure 1A), and 29% (95% CI, 17-42) and 48% (95% CI, 28-88) in HLA class I and class II mismatched patients, respectively (P = .09) (Table 2; supplemental Figure 1). No patient developed grade 4 acute GVHD, and grade 3 acute GVHD was only observed in one HLA class I mismatched patient. No patient developed acute GVHD after day 100. Acute GVHD organ stages are summarized in supplemental Table 2. The cumulative incidence of chronic GVHD was 37% (95% CI, 45-69) and 57% (95% CI, 26-48) at 1 and 4 years (Figure 1B). When separated according to HLA mismatch, the cumulative incidence of chronic GVHD at 1 and 4 years was 44% (95% CI, 30-58) and 63% (95% CI, 49-77) for HLA class I mismatches, and 24% (95% CI, 7-41) and 46% (95% CI, 26-66) for HLA class II mismatches (P = .11) (supplemental Figure 2). The 5-year cumulative incidence of stopping all systemic immunosuppressive treatment was 24% (95% CI, 13-35). No difference was observed in the incidence of grade 2 to 4 acute GVHD or grade 3 to 4 acute GVHD between patients with HLA-DQ mismatches and non–HLA-DQ mismatches.
GVHD. Cumulative incidence of acute (A) and chronic (B) GVHD. In panel A, there were no patients censored before day 100.
GVHD. Cumulative incidence of acute (A) and chronic (B) GVHD. In panel A, there were no patients censored before day 100.
NRM findings in all patients at day 100, 1 year, and 4 years were 4% (95%, CI 0-8), 15% (95% CI, 7-23), and 18% (95% CI, 9-27), respectively (Table 2; supplemental Figure 3), with no difference between HLA class I and II mismatches (class I vs class II, P = .58). Fourteen (18%) patients died of nonrelapse causes: 6 due to infection, 4 due to GVHD, 3 due to GVHD complicated by infection, and 1 due to brain injury from pretransplant radiation therapy for chloroma.
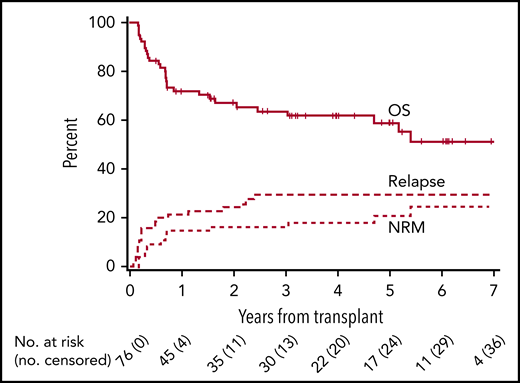
The cumulative incidence of relapse or progression at 1 and 4 years was 21% (95% CI, 12-30) and 30% (95% CI, 19-40), respectively, in all patients (Figure 2). Progression-free survival in all patients at 1 and 4 years was 64% (95% CI, 53-75) and 52% (95% CI, 41-64); overall survivals at the same time points were 72% (95% CI, 61-82) and 62% (95% CI, 50-73). No differences were observed between class I and II mismatches for relapse incidence, progression-free survival, or overall survival (Table 2; supplemental Figures 3 and 4).
Relapse, progression-free survival, and overall survival. Cumulative incidence of relapse, progression-free, and overall survival.
Relapse, progression-free survival, and overall survival. Cumulative incidence of relapse, progression-free, and overall survival.
The cumulative incidence of grade 3 or 4 non-hematopoietic adverse events by day 100 posttransplantation was 29% (95% CI, 19-40). Grades 3 and 4 adverse events are summarized in Table 3. The most common grade 4 adverse event was infection or neutropenic fever. Two (3%) patients experienced a grade 5 fatal event due to pneumonia and multiorgan failure in association with sepsis. One patient experienced transplantation-associated thrombotic microangiopathy. No patients developed Common Terminology Criteria for Adverse Events grade 3 or 4 hypertriglyceridemia or veno-occlusive disease of the liver.
The cumulative incidence of bacterial, fungal, cytomegalovirus, and other viral infections according to HLA class I or II mismatch is summarized in Table 2.
Discussion
The current trial achieved its primary end point by successfully showing that the addition of sirolimus to cyclosporine and mycophenolate mofetil reduced the incidence of grade 2 to 4 acute GVHD after HLA-mismatched HCT from a historical incidence of 69% (observed after class I mismatches) to 34%; the study cohort included HLA class I mismatches as well as class II mismatches that traditionally are associated with very high GVHD rates. Compared with our previous phase 1/2 trial in HLA class I mismatched recipients given GVHD prophylaxis with cyclosporine and mycophenolate mofetil,6 not only was the day 100 incidence of grade 2 to 4 acute GVHD reduced from 69% but the incidence of grade 3 to 4 acute GVHD also decreased (from 26% to 1%). As a result, the NRM declined from 47% to 17% at 4 years’ posttransplantation. The lower NRM was not accompanied by an increased cumulative incidence of relapse or progression, which was 31% after 4 years; this outcome did not differ from the 26% incidence seen in the previous study,6 thus translating into a superior cumulative incidence of progression-free survival (52% vs 31%) and overall survival (62% vs 29%). However, the 4-year incidence of chronic GVHD of 57% was similar in the 2 trials, with a cumulative incidence of patients being able to stop all systemic immunosuppression at 24% at 5 years. Development of chronic GVHD was observed during the taper of cyclosporine and sirolimus, which could support a method of either prolonging the duration of immunosuppressive therapy or other strategies such as the use of posttransplant cyclophosphamide in place of mycophenolate mofetil, which we are currently investigating in a phase 2 randomized clinical trial (#NCT03246906).
Overall, adding sirolimus to cyclosporine and mycophenolate mofetil after HLA class I or II mismatched HCT was well tolerated, with a cumulative incidence of grade 3 to 4 adverse events and infections comparable to what has been observed after HLA-matched HCT.13 Adverse events associated with sirolimus, such as hypertriglyceridemia and veno-occlusive disease of the liver, were not observed, and transplantation-associated thrombotic microangiopathy was observed in only 1 patient.
Outcomes after HLA-mismatched donor HCT in the context of reduced-intensity conditioning have mainly been addressed in registry-based studies14-16 ; observed ranges of grade 2 to 4 acute and chronic GVHD were similar to the current trial, but the incidence of grade 3 to 4 acute GVHD was higher, ranging from 13% to 27%, and resulting in NRM between 31.9% and 39% and overall survival rates between 30% and 48% at 3 years’ posttransplantation. Parody et al17 investigated the efficacy of sirolimus in combination with tacrolimus after reduced-intensity HCT in a prospective study in which a subpopulation of patients (n = 20) was mismatched for a single HLA antigen. In this study, day +100 incidence of grade 2 to 4 acute GVHD was high at 68% but there was a low incidence of grade 3 to 4 acute GVHD at 4.5%. This resulted in survival rates comparable to our current study. Kasamon et al18 combined sirolimus with mycophenolate mofetil and posttransplantation cyclophosphamide as GVHD prophylaxis in a prospective study including 19 patients given 1 to 5 HLA antigen–mismatched grafts after nonmyeloablative conditioning. Results were encouraging, with a cumulative incidence of grade 2 to 4 acute GVHD ranging from 11% to 38% depending on the degree of HLA mismatch, 28% chronic GVHD, 6% NRM, 35% relapse, and 62% overall survival at 3 years’ posttransplantation.
In vivo T-cell depletion with anti-thymocyte globulin (ATG), which is considered the standard of care in HLA antigen–mismatched HCT after myeloablative conditioning, has not been investigated systematically in antigen-mismatched HCT after both truly nonmyeloablative or reduced-intensity conditioning. In a recent review from the European Society of Blood and Marrow Transplantation, it was concluded that ATG prevents GVHD after reduced-intensity conditioning.19 However, risk of relapse was highly dependent on ATG dose, conditioning intensity, and relapse risk of the specific disease, and that due to conflicting results from the currently available data (mainly from retrospective and nonrandomized studies), definite recommendations could not be made. In a matched pair analysis between GVHD prophylaxis with ATG and posttransplantation cyclophosphamide after single HLA antigen–mismatched transplantation based on registry data from the European Society of Blood and Marrow Transplantation, the rate of chronic GVHD was similar between the 2 different prophylactic approaches.20 However, survival measures were overall in favor of posttransplantation cyclophosphamide due to a lower incidence of acute GVHD translating into lower NRM without increasing relapse incidence. Currently, most (63%) unrelated donor HCTs in the United States are performed with calcineurin inhibitor–based regimens; only 33% and 17%, respectively, use ATG-based or posttransplantation cyclophosphamide–based immunosuppression (unpublished data, Center for International Blood and Marrow Transplant Research, 2019).
With the introduction of posttransplantation cyclophosphamide, HLA-haploidentical transplantation has become a viable treatment option.21,22 Due to the low rates of chronic GVHD and NRM observed with this regimen, transplantation centers are using HLA haploidentical donors with increasing frequency,23,24 thereby challenging the donor selection algorithm of single HLA antigen–mismatched donors being first choice if fully HLA-matched or single allele HLA level mismatched donors are not available. Furthermore, side-by-side comparison of GVHD rates between haploidentical and mismatched unrelated donor transplant cohorts is complicated by the use of different GVHD prophylaxis regimens. The change in practice is based mostly on registry data reporting the incidence of grade 2 to 4 acute GVHD between 20% and 45% and low rates of chronic GVHD ranging from 12% to 38%, resulting in NRM rates between 6% and 24% and overall survival rates between 38% and 63%.25-28 Prospective data are few and from smaller studies that, in general, validate the findings of a low incidence of chronic GVHD and NRM; however, this benefit has been offset by high relapse rates ranging from 24% to 55%.29-31 A recent meta-analysis that included a total of 30 studies with 22 974 patients suggested that matched related donors were still to be considered optimal, but that HLA-haploidentical HCT with posttransplantation cyclophosphamide was associated with an overall survival advantage compared with single HLA antigen–mismatched HCT due to lower NRM and similar incidence of relapse.32 The question of whether HLA-haploidentical HCT with posttransplantation cyclophosphamide should be preferred over single HLA antigen–mismatched HCT can only be answered in a direct comparison under the auspices of a randomized trial. In HLA-haploidentical HCT with posttransplantation cyclophosphamide as GVHD prophylaxis, sirolimus has been investigated in combination with mycophenolate mofetil in the setting of myeloablative conditioning.33 The prospective trial included 40 patients and reported a cumulative incidence of 15% and 7.5% for grade 2 to 4 acute GVHD and grade 3 to 4 acute GVHD at day 100 posttransplantation, respectively, with a low incidence of chronic GVHD at 20% after 1 year. NRM and relapse incidence were 17% and 35%, resulting in an overall survival of 56% at 1 year posttransplantation.
Although results are encouraging, and improved results have been observed compared with our earlier trial,6 the efficacy of the investigated combination of immunosuppressive drugs has to be tested against other novel strategies used for HLA-mismatched HCT. No differences were observed between patients who are mismatched at HLA class I or II. However, because the trial was underpowered to detect any differences in outcome between HLA class I or II mismatches, these findings have to be validated in an adequately powered trial.
In conclusion, the current trial shows that addition of sirolimus to GVHD prophylaxis with cyclosporine and mycophenolate mofetil in HLA class I or II mismatched donor HCT after nonmyeloablative conditioning is safe and capable of reducing acute GVHD and NRM without increasing the risk of relapse or progression in older or medically infirm patients. Our results compare positively to what we previously observed in a randomized phase 3 trial with the same combination of immunosuppressive drugs after HLA-matched unrelated donor HCT following nonmyeloablative conditioning resulted in less grade 2 to 4 acute GVHD and NRM, and superior rates in overall and progression-free survival.13 These findings suggest that the immunomodulatory effects of sirolimus may overcome the otherwise deleterious consequences of HLA mismatching. Although results of the current study are encouraging, no reduction in the incidence of chronic GVHD was observed, leaving open the question of approaches to reduce the risk of chronic GVHD. In addition to the nonmyeloablative conditioning regimen used in the current study, other reduced-intensity regimens have proven successful in HLA antigen–mismatched transplantation. Between 33% and 50% of patients in the reports by Gaballa et al30 and Battipaglia et al20 received reduced-intensity conditioning, mainly based on fludarabine in combination with low-dose TBI, busulfan, or melphalan. To address the question of chronic GVHD, we are conducting a phase 2 randomized trial (#NCT03246906) comparing mycophenolate mofetil, cyclosporine, and sirolimus with posttransplantation cyclophosphamide, cyclosporine, and sirolimus in unrelated HLA-matched or single antigen-mismatched PBSC HCT after conditioning with FM100 (fludarabine 120 mg/m2, melphalan 100 mg/m2 and 2 Gy TBI), FluBu2 (fludarabine 150 mg/m2 and busulfan 6.4 mg/kg IV or 8 mg/kg by mouth), or Flu/TBI (fludarabine 90 mg/m2 and 2-3 Gy TBI).
Individual participant data will not be shared.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in the clinical trial. They also thank their colleagues, clinical staff on the transplant services, the research staff, and the referring physicians at all the participating sites. The authors also thank Derek Stirewalt and the rest of the Data and Safety Monitoring Board members for their review of the conduct of the trial. They greatly appreciate Helen Crawford’s assistance with manuscript and figure preparation.
Research funding was provided by the National Institutes of Health, National Cancer Institute (P01 CA018029, P01 CA078902, and P30 CA015704). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its subsidiary institutes.
Authorship
Contribution: B.K. drafted the protocol, drafted and revised the manuscript, and analyzed and interpreted data; B.E.S. designed the study, performed statistical calculations, and revised the manuscript; N.S.A., M.B.M., T.R.C., E.W.P., A.E.W., and M.E.D.F. provided patients and revised the manuscript; R.S. designed the study, provided funding, provided patients, and revised the manuscript; D.G.M. designed the study, reviewed enrollment of patients, provided patients, and revised the manuscript; and B.M.S. conceived, designed, and supervised the study, reviewed enrollment of patients, provided patients, analyzed and interpreted data, drafted and revised the manuscript, and provided funding.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brenda M. Sandmaier, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, P.O. Box 19024, Seattle, WA 98109-1024; e-mail: bsandmai@fredhutch.org.