The mechanisms by which glucocorticoid treatment leads to rapid reduction of circulating eosinophils is poorly understood. In this issue of Blood, Hong et al provide new experimental evidence that CXCR4-mediated eosinophil homing to the bone marrow is a relevant pathway for this process.1
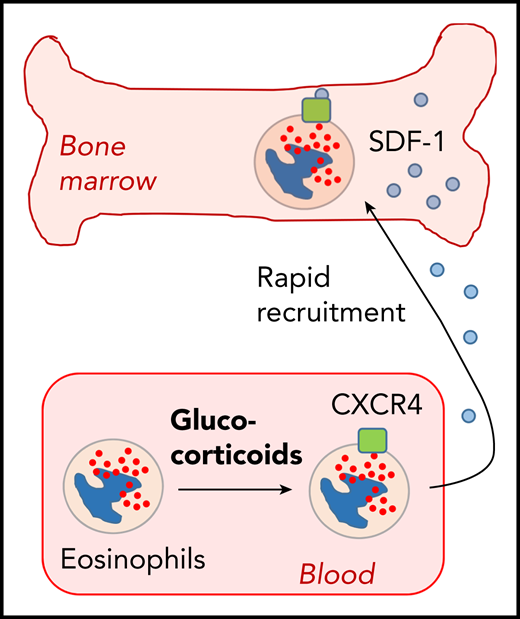
Hong et al show that glucocorticoid-induced expression of CXCR4 in blood eosinophils leads to their rapid recruitment to the bone marrow.
Hong et al show that glucocorticoid-induced expression of CXCR4 in blood eosinophils leads to their rapid recruitment to the bone marrow.
Eosinophils are potent effector cells of the innate immune system and contribute to protection against helminth infections. However, they can also cause severe damage during allergic responses, eosinophil-associated chronic inflammation, and hypereosinophilic syndromes.2 The development of eosinophils occurs in the bone marrow from granulocyte-macrophage (GM) progenitors, which first differentiate to eosinophil progenitors and then to mature eosinophils under control of various transcription factors, including GATA-1, Helios, and Aiolos.3,4 Mature eosinophils rapidly leave the bone marrow and reach sites of inflammation via blood circulation and extravasation into tissues. Their survival is mainly promoted by interleukin-5 (IL-5) and GM colony stimulating factor, which can be produced in large amounts in inflamed tissues and lead to upregulation of the antiapoptotic molecule Bcl-xL.5
Although new therapeutic options for specific targeting of eosinophils are being developed, glucocorticoids have been used for decades for efficient induction of eosinopenia in humans, despite their significant toxicity.6 Eosinopenia occurs within a few hours, but the molecular mechanisms behind the disappearance of eosinophils from blood circulation are poorly defined. Interestingly, glucocorticoids do not induce eosinopenia in mice, making mouse models unsuitable. Nagase et al have shown that dexamethasone induces upregulation of the chemokine receptor CXCR4 on eosinophils, but not on neutrophils.7 This suggested that glucocorticoids might induce sequestration of eosinophils from the blood circulation by attracting them to extravascular sites. However, it was unclear where eosinophils actually go after glucocorticoid treatment. Hong et al used whole-body in vivo imaging of radioactivity-labeled and transferred eosinophils in rhesus macaques. They followed the distribution of transferred eosinophils for ≤4 hours after transfer by positron emission tomography/computed tomography scanning. Within the first 10 minutes after transfer, eosinophils were mainly found in the lung. This picture changed after 1 hour, when transferred eosinophils were dominantly present in the bone marrow, and further increased for ≤4 hours in this compartment. Eosinophil accumulation was not observed in other organs like liver, lung, and spleen. The kinetics of eosinophil homing to the bone marrow correlated with increasing expression levels of CXCR4 and blocking this receptor prevented the disappearance of eosinophils from the blood circulation. These findings indicate that glucocorticoid-induced eosinopenia results from CXCR4-regulated homing of peripheral eosinophils back to the bone marrow within a few hours. Whether these eosinophils then die in the bone marrow or recirculate back to the bloodstream needs to be determined.
Which other potential mechanisms could be involved in glucocorticoid-induced eosinopenia? Glucocorticoids have a broad spectrum of target cells, including nonhematopoietic cell types. For example, they have been described to induce expression of adhesion molecules in endothelial cells, and this effect may to some extent contribute to the rapid and efficient reduction in blood eosinophil counts.8 Furthermore, glucocorticoids promote expression of several proapoptotic genes in eosinophils, indicating that induction of cell death could also be involved as a potential mechanism.9 Further studies on mechanisms of glucocorticoid-mediated eosinopenia may help to refine therapeutic treatments of eosinophil-associated disorders, including combination therapies with more eosinophil-specific targets, such as anti-IL-5 or anti-IL-5 receptor blockade.
Conflict-of-interest disclosure: The author declares no competing financial interests.