Key Points
Monoclonal immunoglobulins of myeloma patients with bone disease have reduced IgG-Fc glycosylation and promote osteoclast differentiation.
Treatment with ManNAc to increase IgG sialylation reduces the number of osteolytic lesions and tumor load in a myeloma mouse model.
Abstract
Most patients with multiple myeloma develop a severe osteolytic bone disease. The myeloma cells secrete immunoglobulins, and the presence of monoclonal immunoglobulins in the patient’s sera is an important diagnostic criterion. Here, we show that immunoglobulins isolated from myeloma patients with bone disease promote osteoclast differentiation when added to human preosteoclasts in vitro, whereas immunoglobulins from patients without bone disease do not. This effect was primarily mediated by immune complexes or aggregates. The function and aggregation behavior of immunoglobulins are partly determined by differential glycosylation of the immunoglobulin-Fc part. Glycosylation analyses revealed that patients with bone disease had significantly less galactose on immunoglobulin G (IgG) compared with patients without bone disease and also less sialic acid on IgG compared with healthy persons. Importantly, we also observed a significant reduction of IgG sialylation in serum of patients upon onset of bone disease. In the 5TGM1 mouse myeloma model, we found decreased numbers of lesions and decreased CTX-1 levels, a marker for osteoclast activity, in mice treated with a sialic acid precursor, N-acetylmannosamine (ManNAc). ManNAc treatment increased IgG-Fc sialylation in the mice. Our data support that deglycosylated immunoglobulins promote bone loss in multiple myeloma and that altering IgG glycosylation may be a therapeutic strategy to reduce bone loss.
Introduction
Multiple myeloma is a hematologic malignancy caused by clonal proliferation of malignant plasma cells in the bone marrow.1 This expansion causes high circulating levels of monoclonal immunoglobulins (M component) in most patients; ∼70% of the patients have serum monoclonal immunoglobulin levels > 2 g/dL. The majority of patients have immunoglobulin G (IgG) myeloma (50%), whereas IgA myeloma and light chain–only myeloma account for 20% and 16% of the cases, respectively.2 Except for kidney damage, the M component is not known to be involved in the pathogenesis of the disease.
One of the hallmarks of multiple myeloma is the presence of osteolytic lesions caused by an increase in numbers and activity of bone-degrading osteoclasts and a decrease in bone-forming osteoblasts. Myeloma bone disease (BD) is a severe clinical problem, causing fractures, pain, and reduced quality of life.3-5 Bone loss is also common in inflammatory autoimmune diseases such as rheumatoid arthritis. In mouse models of rheumatoid arthritis, autoantibodies in the form of immune complexes have been shown to promote bone loss.6-8 A conserved glycosylation site is present in the CH2 domain of the IgG heavy chain, and the type of glycan at this site is critical for conformation of the Fc part as well as for the binding affinity to various Fcγ receptors (FcγRs).9 The pro-osteoclastogenic effect of IgGs in rheumatoid arthritis seems to be, at least partly, dependent on altered glycosylation of the IgG-Fc part.10,11
Multiple myeloma is not considered an autoimmune disease, and the antigens underlying the origin of myeloma clones are to a large extent unknown. A recent article showed that monoclonal and polyclonal immunoglobulins from myeloma patients have reduced Fc-sialylation compared with immunoglobulins from healthy persons.12 In addition, we recently reported a decrease in both sialylation and galactosylation in the total serum protein N-glycome in myeloma patients compared with healthy control subjects.13 It remains to be investigated whether glycosylation patterns of immunoglobulins differ in myeloma patients with and without BD. Considering the high levels of circulating immunoglobulins and changes in immunoglobulin Fc-glycosylation in myeloma patients, we sought to investigate if immunoglobulins play a role in multiple myeloma BD.
Methods
Patient samples
All samples were donated after informed consent, and the regional ethics committee (REK 2011/2029 and 2012/2033) approved the study. For osteoclast assay and glycosylation analysis, patient serum samples were obtained from Biobank1 (St. Olavs Hospital, Trondheim, Norway). The serum samples were collected between 2005 and 2015 and had been stored by the Biobank at −80°C. In addition, serum samples obtained in 2013 from 51 age- and sex-matched healthy donors were used. No outliers were excluded from the analyses. Patient characteristics are presented in Tables 1 and 2. Bone affection was determined by radiographs unless otherwise stated (supplemental Table 1, available on the Blood Web site). Patients with BD had significantly higher serum CTX-1 compared with healthy control subjects and patients without BD (supplemental Figure 1). Additional details are provided in the supplemental Methods.
Isolation and fractionation of immunoglobulins
Isolation of immunoglobulins was performed by using G- and L-protein magnetic beads (Thermo Fisher Scientific/Life Technologies) or protein G SpinTrap (GE Healthcare) according to the manufacturers’ instructions. Protein measurements of the isolated products were performed by using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific) or the NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies Inc.) according to the manufacturers’ instructions. Isolated immunoglobulins were fractioned by gel filtration using a Superose 6 3.2/300 column (GE Healthcare). Fractions were collected in 100 μL of phosphate-buffered saline and stored at 4°C until used.
IgG glycosylation analysis of human and mouse samples
Mass spectrometric analyses of human and mouse IgG glycosylation were performed at the Center for Proteomics and Metabolomics, Leiden University Medical Center. Details are provided in the supplemental Methods.
Mass spectrometric analyses of IgG-binding proteins
IgG isolated from a patient’s serum as described earlier was analyzed on an Orbitrap Elite (Thermo Fisher Scientific) to identify binding proteins. The mass spectrometric analysis was performed at the Proteomics Unit at University of Bergen. Details are provided in the supplemental Methods.
Enzymatic modifications of isolated immunoglobulins
Removal of sialic acid and galactose was performed by incubating isolated IgG with 10 U of α2-3,6,8,9 neuraminidase A and 8 U of β1-4 galactosidase in 1X GlycoBuffer (New England BioLabs) for 24 hours at 37°C. Addition of galactose was performed by incubating immunoglobulin (1 mg) with UDP-galactose (10 µM; MilliporeSigma) and β1-4 galactosyltransferase (2.5 mU; MilliporeSigma) in 4-morpholinepropanesulfonic acid buffer (50 mM pH 7.2 with 20 mM MnCl2) for 48 hours at 37°C. The enzymatically modified immunoglobulins were purified over Zeba Spin columns (Thermo Fisher Scientific).
Nanoparticle tracking analysis of immunoglobulins
Isolated immunoglobulins were diluted in sterile phosphate-buffered saline and analyzed by using NanoSight NS300 (Malvern Panalytical). Particle size and concentrations were calculated in NTA3.2 Dev Build 3.2.16. The detection threshold was set to 5.
Differentiation of osteoclasts and TRAP staining
Human buffy coats for isolation of peripheral blood mononuclear cells (PBMCs) used for osteoclast assays were provided by the blood bank at St. Olavs Hospital (approved REK, no. 2009/2245). Isolation of PBMCs from buffy coats was performed with density gradient (Lymphoprep; Axis-Shield Diagnostics Ltd.), and CD14+ cells were isolated by using magnetic beads (MACS; Miltenyi Biotec) as previously described.14 Isolated monocytes (67.000 CD14+ cells/mL) were cultured in α−minimum essential medium (without phenol red) (Thermo Fisher Scientific) with pooled human serum (10%) and macrophage colony-stimulating factor (30 ng/mL; R&D Systems), RANKL (20 ng/mL; R&D Systems) and transforming growth factor-β (1 ng/mL; R&D Systems) for 6 to 10 days until binuclear cells appeared. At that point, macrophage colony-stimulating factor (30 ng/mL), RANKL (10 ng/mL), and isolated immunoglobulins (5 µg/mL) were added as specified in Figure 1. Cells were stained with TRAP by using the Tartrate-Resistant Acid Phosphatase, Leukocyte (TRAP) Kit (MilliporeSigma) according to the manufacturer’s instructions, with the following modifications: Cells were fixed using paraformaldehyde (4%), and the samples were incubated in the staining solution for up to 2 hours. TRAP-positive multinuclear cells with ≥3 nuclei were regarded as osteoclasts and counted blinded under standard phase-contrast microscopy.
IgG isolated from myeloma patients with BD induce osteoclastogenesis. (A-B) Immunoglobulins isolated from patients with BD (BD) or without BD (no BD) or from healthy control subjects were added to preosteoclasts derived from CD14+ cells isolated from PBMCs of healthy donors. After 2 to 4 days, cells were TRAP stained, and TRAP-positive cells with ≥3 nuclei were counted as osteoclasts. Cells were incubated with RANKL (10 ng/mL) as a positive control and macrophage colony-stimulating factor (M-CSF; 30 ng/mL) only as a negative control In panel A, IgG from IgG-myeloma patients (the M component) (5 μg/mL) with (n = 16) and without (n = 10) BD, were used; in panel B, IgGs (5 μg/mL) isolated from the serum of IgA, IgD or nonsecretory patients (uninvolved immunoglobulins) with (n = 17) and without (n = 10) BD were used. Numbers of osteoclasts are presented relative to the RANKL positive control. **P < .01 using ANOVA followed by the Šidák multiple comparisons test. (C) Relative increase in osteoclasts after immunoglobulins (5 μg/mL, n = 4 patients with BD) were added to preosteoclasts that were previously blocked with neutralizing anti-FcγR antibodies. The number of TRAP-positive cells was counted after 1 to 3 days. **P < .01 using mixed model ANOVA followed by Tukey’s multiple comparisons test. (D) Preosteoclasts were stimulated with serum from myeloma patients (n = 4) with and without FcγRII antibody. The number of osteoclasts after 1 to 2 days was evaluated by using TRAP staining. ***P < .001, ****P < .0001 using ANOVA followed by the Šidák multiple comparisons test. (E-F) Isolated immunoglobulins (n = 3 per group) were separated based on size on a Sepharose 6 size-exclusion column, and the different fractions were added to preosteoclasts. The number of mature osteoclasts after 1 to 3 days was evaluated by using TRAP staining. **P < .01 using repeated measurements one-way ANOVA followed by Dunnett’s multiple comparisons test. (G) Amount of nanoparticles over 100 nm (range, 50-200 nm) in size for healthy control subjects (n = 11), no-BD patients (n = 18), and BD patients (n = 20). Error bars represent the standard error of the mean. *P < .05 using ANOVA followed by the Šidák multiple comparisons test. (H) Mass spectrometric analyses of IgG derived from patients with (n = 11) or without (n = 8) BD. P values corrected for multiple comparisons are shown on the y-axis. ns, not significant.
IgG isolated from myeloma patients with BD induce osteoclastogenesis. (A-B) Immunoglobulins isolated from patients with BD (BD) or without BD (no BD) or from healthy control subjects were added to preosteoclasts derived from CD14+ cells isolated from PBMCs of healthy donors. After 2 to 4 days, cells were TRAP stained, and TRAP-positive cells with ≥3 nuclei were counted as osteoclasts. Cells were incubated with RANKL (10 ng/mL) as a positive control and macrophage colony-stimulating factor (M-CSF; 30 ng/mL) only as a negative control In panel A, IgG from IgG-myeloma patients (the M component) (5 μg/mL) with (n = 16) and without (n = 10) BD, were used; in panel B, IgGs (5 μg/mL) isolated from the serum of IgA, IgD or nonsecretory patients (uninvolved immunoglobulins) with (n = 17) and without (n = 10) BD were used. Numbers of osteoclasts are presented relative to the RANKL positive control. **P < .01 using ANOVA followed by the Šidák multiple comparisons test. (C) Relative increase in osteoclasts after immunoglobulins (5 μg/mL, n = 4 patients with BD) were added to preosteoclasts that were previously blocked with neutralizing anti-FcγR antibodies. The number of TRAP-positive cells was counted after 1 to 3 days. **P < .01 using mixed model ANOVA followed by Tukey’s multiple comparisons test. (D) Preosteoclasts were stimulated with serum from myeloma patients (n = 4) with and without FcγRII antibody. The number of osteoclasts after 1 to 2 days was evaluated by using TRAP staining. ***P < .001, ****P < .0001 using ANOVA followed by the Šidák multiple comparisons test. (E-F) Isolated immunoglobulins (n = 3 per group) were separated based on size on a Sepharose 6 size-exclusion column, and the different fractions were added to preosteoclasts. The number of mature osteoclasts after 1 to 3 days was evaluated by using TRAP staining. **P < .01 using repeated measurements one-way ANOVA followed by Dunnett’s multiple comparisons test. (G) Amount of nanoparticles over 100 nm (range, 50-200 nm) in size for healthy control subjects (n = 11), no-BD patients (n = 18), and BD patients (n = 20). Error bars represent the standard error of the mean. *P < .05 using ANOVA followed by the Šidák multiple comparisons test. (H) Mass spectrometric analyses of IgG derived from patients with (n = 11) or without (n = 8) BD. P values corrected for multiple comparisons are shown on the y-axis. ns, not significant.
FcγR inhibition
Cells were seeded out, cultured, and induced to differentiate toward osteoclasts as described until binuclear cells appeared. At this point, cells were treated with blocking antibodies (10 µg/mL) against FcγRI (10.1; BioLegend), FcγRII (AT10; Abcam), FcγRIII (BioLegend), or isotype control (mouse IgG1, κ; BioLegend) for 1 hour. Next, immunoglobulins (10 µg/mL) were added as indicated in Figure 1, and cells were cultured for up to 5 additional days before TRAP staining as described. In the serum experiments, FcγRII-neutralizing antibodies were added to binuclear cells 30 minutes before stimulation with human serum (30%) from individual myeloma patients. Cells were cultured 1 to 2 days until multinuclear cells appeared and thereafter were TRAP stained.
5TGM1 mouse model
The mouse experiment was performed at Pharmatest Services Ltd. and was approved by the National Committee for Animal Experiments in Finland. Female C57BL/KaLwRij mice, aged 6 to 7 weeks at the beginning of the study, were used. On study day 0, a total of 20 mice were given an intravenous injection of 2 × 106 of 5TGM1 mouse multiple myeloma cells. Allocation to treatment groups (n = 10) was performed by using a stratification procedure based on the animal weight at the beginning of the study.
The treatment with N-acetylmannosamine (ManNAc; MilliporeSigma) and the corresponding control mannose, both administered at 10 g/L in the drinking water, was initiated on study day 0, within 1 to 2 hours’ postinoculation. For the duration of the study, the overall liquid consumption was monitored, and the weight of each bottle was registered. Blood samples were collected 1 day before euthanasia or at end point. To minimize the inbetween subjects’ variability in bone marker measurements, blood was collected in the afternoon after 4 hours of fasting, when possible. The number of bone lesions was evaluated by radiographic imaging of both hind limbs, and the bone microstructure was evaluated by micro–computed tomography analysis of the right femur, as described in the supplemental Methods. Tumor load was assessed by quantification of serum IgG2b levels (M component), spleen weight, and percent plasma cells in bone marrow.
Statistical analysis
Statistical analyses were performed by using Graph Pad Prism 8 and SPSS version 24 for Mac OS X. The Student t test was used to determine significance between 2 groups, and one-way analysis of variance (ANOVA) followed by the Šidák multiple comparisons test were used to determine significance when comparing multiple factors. For paired analyses, repeated measurements or mixed models ANOVA followed by Tukey’s or Dunnett’s multiple comparisons were used. Correlation between 2 parameters was estimated by the Spearman rank correlation analysis. For non-normally distributed parameters: (1) when comparing 2 groups, the Mann-Whitney U test was used; and (2) when comparing multiple groups, the Kruskal-Wallis test, followed by the Dunnett multiple comparisons test, was used. For paired analyses, the Wilcoxon matched-pairs signed rank test was used. Results were considered statistically significant when P < .05.
Results
Immunoglobulins from multiple myeloma patients with BD promoted osteoclastogenesis in vitro
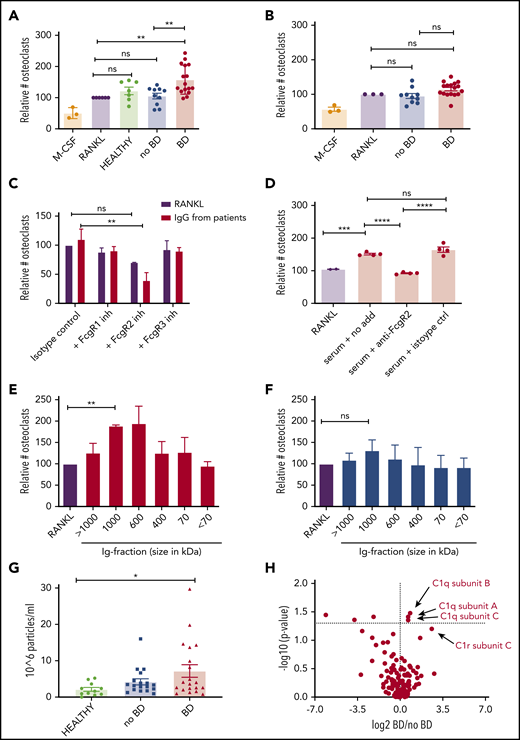
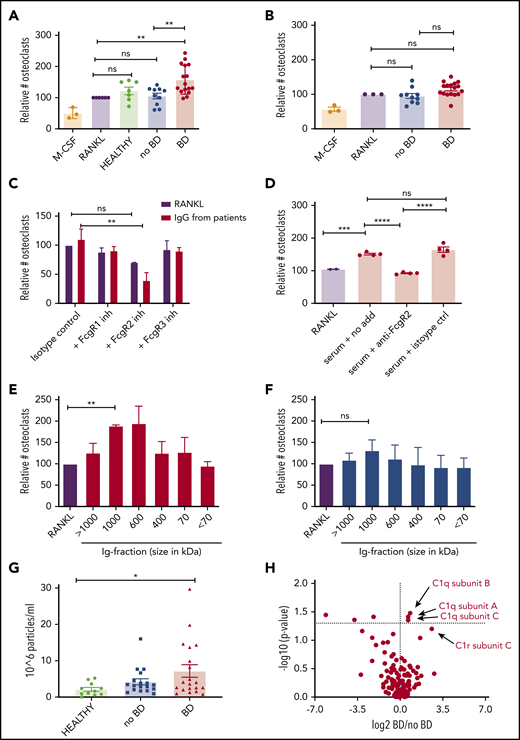
To investigate whether immunoglobulins from myeloma patients influence osteoclastogenesis, we first isolated IgG from the serum of myeloma patients who had IgG M component with or without BD, added them to preosteoclasts generated from PBMCs, and quantified the number of osteoclasts. Strikingly, immunoglobulins isolated from patients with BD (n = 16) induced osteoclast formation, whereas immunoglobulins isolated from healthy control subjects (n = 7) or patients without BD (n = 10) had no effect relative to the RANKL control (Figure 1A; supplemental Figure 2A). In contrast, when IgG was isolated from patients with IgA, IgD, or nonsecretory myeloma, the “normal” or so-called uninvolved immunoglobulins, IgG from these patients did not promote osteoclastogenesis, regardless of the BD status of the patient (Figure 1B). These data suggest that the pro-osteoclastogenic effect is mediated by the M component and not by the uninvolved immunoglobulins. IgG isolated from blood promoted osteoclast differentiation to a similar extent as IgG isolated from bone marrow plasma from the same patient (supplemental Figure 2B). The clinical characteristics of the patients are summarized in Table 1 and supplemental Table 1.
To investigate if the observed effect on osteoclastogenesis was dependent on Fc-receptors, we inhibited FcγRI, FcγRII, and FcγRIII on preosteoclasts using neutralizing antibodies. When FcγRII was inhibited, osteoclastogenesis was reduced compared with the isotype control (Figure 1C). Inhibiting FcγRI or FcγRIII had no effect, suggesting that FcγRII is important for mediating the effect of the isolated immunoglobulins on preosteoclasts. To further examine the relative importance of FcγRII for immunoglobulin-mediated osteoclastogenesis, we added serum from myeloma patients with BD to preosteoclasts and inhibited FcγRII using neutralizing antibodies. Indeed, inhibiting FcγRII significantly reduced osteoclastogenesis compared with isotype control-treated cells (Figure 1D), suggesting that immunoglobulins to a large extent mediate the pro-osteoclastogenic effect of myeloma patients’ serum.
Because FcγRII is a low-affinity receptor for monomeric immunoglobulins, we wanted to examine if immune complexes or aggregated immunoglobulins mediated the effect on osteoclasts. To this end, we fractioned the isolated immunoglobulins using gel filtration and stimulated preosteoclasts with the different molecular weight fractions. Indeed, for patients with BD, the fractions containing immune complexes of ∼600 to 1000 kDa contained the “active” components (Figure 1E). In contrast, for patients without BD, there was little or no effect of immunoglobulins on osteoclastogenesis, even in the fractions containing immune complexes (Figure 1F). Monomeric immunoglobulins (∼150 kDa) had no effect compared with the control, implying that immune complexes or aggregates (and not monomeric immunoglobulins) are the culprits for the osteoclastogenic effect. In an attempt to estimate the amount of complexes/aggregates in the samples, we quantified the number of particles with a diameter of ≥100 nm in our isolated immunoglobulin fractions using nanoparticle tracking analysis. We found that samples from patients with BD contained more nanoparticles compared with the healthy control subjects, whereas there was a nonsignificant difference between patients with or without BD (Figure 1G). To further characterize differences between the groups, mass spectrometric analyses were performed of immunoglobulins isolated from patients with (n = 11) and without (n = 8) BD. The only proteins that were significantly upregulated in the BD group were C1q subcomponent subunit B, C1q subcomponent subunit A, and C1q subcomponent subunit C. This finding indicates that immunoglobulins from patients with BD bind more complement C1q compared with patients without BD (Figure 1H).
Reduced Fc-glycosylation of immunoglobulins from multiple myeloma patients with BD
Complex formation/aggregation and receptor binding is influenced by Fc-glycosylation of immunoglobulins.9 To examine if there were changes in Ig-glycosylation in myeloma patients with BD, IgG Fc-glycosylation was characterized by mass spectrometry of immunoglobulins isolated from myeloma patients with (n = 43) and without (n = 33) BD and from healthy control subjects (n = 51) (Table 2). The derived glycosylation traits in the 3 groups are presented in a heat map (Figure 2A). It is evident from the map that patients with BD have decreased immunoglobulin galactosylation and sialylation compared with patients without BD (supplemental Table 2). These differences were mainly caused by a decrease in galactosylation and to some degree sialylation, as is seen in the lower part of the heat map. According to a more detailed analysis, a significant reduction in sialylation was noted in patients with BD compared with healthy control subjects (Figure 2B), as well as a reduction in galactosylation in patients with BD compared with patients without BD and with healthy control subjects (Figure 2C). For other traits, such as core fucosylation or bisection, there was no difference between patients with or without BD. However, fucosylation was significantly higher in myeloma patients compared with healthy control subjects (supplemental Figure 3).
Glycosylation of immunoglobulins differs between myeloma patients with and without BD. (A) Heat map presenting glycosylation traits in the 3 groups: healthy control subjects (n = 51), no BD (n = 33), and BD (n = 43). TC, total complex; F, fucosylation; B, bisection; G, galactosylation; S, sialylation; S0, nonsialylated; F0, nonfucosylated; G0, nongalactosylated; A2, Diantennary glycans. The last letter indicates the subject of the calculation (eg, fucosylation [F]), and the preceding letters indicate the group on which it is calculated. Sialylation (B) and galactosylation (C) of immunoglobulins in healthy control subjects (n = 51), myeloma patients without BD (n = 33), and patients with BD (n = 43). *P < .05, **P < .01 using the Kruskal-Wallis test, then Dunnett’s multiple comparisons. Sialylation (D) and galactosylation (E) of individual patients before and after the onset of BD (n = 8). *P < .05 using Wilcoxon matched-pairs signed rank test. (F) Serum CTX-1 levels in patients with low (n = 17, lower 20th percentile) or high (upper 20th percentile) IgG galactosylation. (G) Volcano plot comparing glycosyltransferase expression in myeloma cells in patients without (n = 36) and with (n = 137) BD. Unadjusted P values are shown on the y-axis. Error bars represent mean ± standard error of the mean. *P < .05, **P < .01 using the Mann-Whitney U test.
Glycosylation of immunoglobulins differs between myeloma patients with and without BD. (A) Heat map presenting glycosylation traits in the 3 groups: healthy control subjects (n = 51), no BD (n = 33), and BD (n = 43). TC, total complex; F, fucosylation; B, bisection; G, galactosylation; S, sialylation; S0, nonsialylated; F0, nonfucosylated; G0, nongalactosylated; A2, Diantennary glycans. The last letter indicates the subject of the calculation (eg, fucosylation [F]), and the preceding letters indicate the group on which it is calculated. Sialylation (B) and galactosylation (C) of immunoglobulins in healthy control subjects (n = 51), myeloma patients without BD (n = 33), and patients with BD (n = 43). *P < .05, **P < .01 using the Kruskal-Wallis test, then Dunnett’s multiple comparisons. Sialylation (D) and galactosylation (E) of individual patients before and after the onset of BD (n = 8). *P < .05 using Wilcoxon matched-pairs signed rank test. (F) Serum CTX-1 levels in patients with low (n = 17, lower 20th percentile) or high (upper 20th percentile) IgG galactosylation. (G) Volcano plot comparing glycosyltransferase expression in myeloma cells in patients without (n = 36) and with (n = 137) BD. Unadjusted P values are shown on the y-axis. Error bars represent mean ± standard error of the mean. *P < .05, **P < .01 using the Mann-Whitney U test.
We next analyzed Fc-glycosylation of IgG from 8 myeloma patients who did not have BD at the time of diagnosis but who later developed BD. Extraordinarily, we found that sialylation decreased in all these patients as they developed BD (Figure 2D). There was also a trend toward reduced galactosylation when BD had manifested, although this difference was not significant (Figure 2E). Moreover, the group of patients with low IgG galactosylation (lower 20th percentile) had significantly higher levels of serum CTX-1, a marker for osteoclast activity, compared with the group of patients with high IgG galactosylation (upper 20th percentile) (Figure 2F). Taken together, our data show a negative association between IgG sialylation and galactosylation and bone loss in multiple myeloma. All derived glycosylation traits are presented in supplemental Table 3.
Reduced immunoglobulin galactosylation and sialylation may be caused by reduced expression of glycosyltransferases in plasma cells.11 To further explore the relation between immunoglobulin glycosylation and BD, we compared the expression of transferases involved in sialic acid, galactose, N-acetylglucosamine, and fucose addition between myeloma patients with and without BD from a publicly available microarray gene expression dataset (GSE755). This data series includes samples from 137 patients with BD and 36 patients without BD. Two transferases, the sialyltransferase ST6GAL1 and the galactosyltransferase B4GALT1, were expressed significantly lower in the BD group compared with the no-BD group (Figure 2G). The data for ST6GAL1 and B4GALT1 are presented in more detail in supplemental Figure 4. Similar results were found in the large CoMMpass data set (interim analysis 11) consisting of RNA-sequencing data from 767 diagnostic samples: both ST6GAL1 and B4GALT1 were significantly downregulated in the BD patients compared with those without BD (supplemental Figure 5A-B). There was also decreasing expression of ST6GAL1 and B4GALT1 with increasing number of lesions (supplemental Figure 5C-D). These data further strengthen the hypothesis that reduced immunoglobulin sialylation and galactosylation are associated with bone loss in myeloma.
Modifications of immunoglobulin glycosylation alter the pro-osteoclastogenic properties
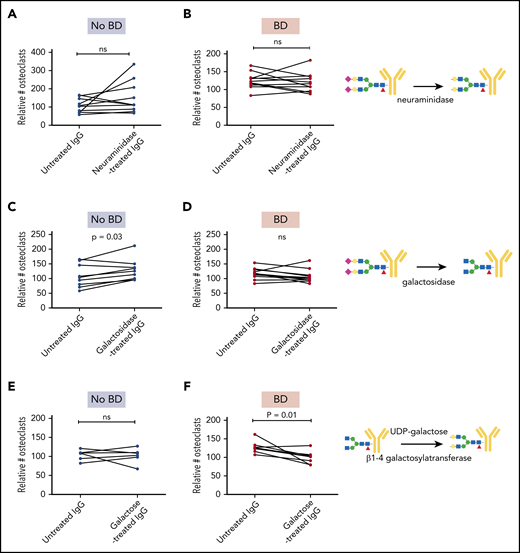
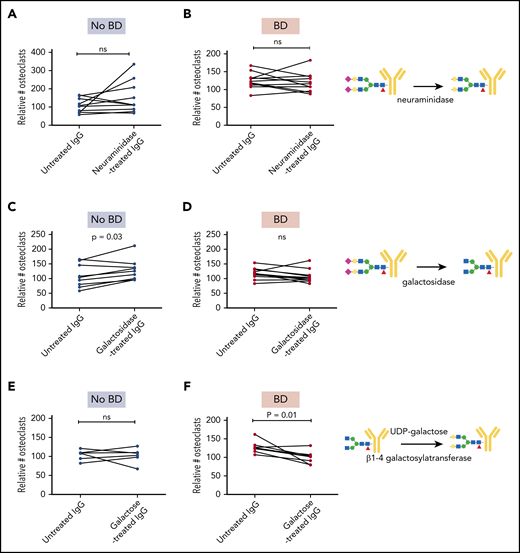
To determine if the glycosylation differences were important for the osteoclast-promoting effect of the myeloma-derived immunoglobulins, we treated immunoglobulins with neuraminidase and galactosidase to remove sialic acid and galactose, respectively. Neuraminidase treatment of immunoglobulins from either group of patients had no significant effect on osteoclastogenesis (Figure 3A-B). Importantly, however, removing galactose from IgG isolated from patients without BD made these immunoglobulins pro-osteoclastogenic (Figure 3C), whereas the same treatment had no effect on immunoglobulins isolated from patients with BD (Figure 3D). A possible explanation for this difference might be that IgGs from patients with BD are lacking galactose to start with, and thus treating them with galactosidase will have no effect.
Enzymatic modifications of immunoglobulins alter pro-osteoclastogenic properties. IgG from individual patients with and without BD (n = 7-11 in each group) were treated with neuraminidase (A-B) and galactosidase (C-D) to reduce sialylation and galactosylation, respectively, and then added to preosteoclasts for 2 to 4 days before the number of TRAP-positive cells with ≥3 nuclei was counted. (E-F) IgG from patients with and without BD were treated with UDP-galactose to increase galactosylation and added to preosteoclasts for 2 to 4 days before the number of TRAP-positive cells with ≥3 nuclei was counted. The number of mature osteoclasts was evaluated by using TRAP stain. Data presented in panels A-F are relative to RANKL control. P values determined by Wilcoxon matched-pairs signed rank test. Symbols: purple diamond, sialic acid; yellow circle, galactose; blue square, GlcNAc; green circle, mannose; red triangle, fucose. ns, not significant.
Enzymatic modifications of immunoglobulins alter pro-osteoclastogenic properties. IgG from individual patients with and without BD (n = 7-11 in each group) were treated with neuraminidase (A-B) and galactosidase (C-D) to reduce sialylation and galactosylation, respectively, and then added to preosteoclasts for 2 to 4 days before the number of TRAP-positive cells with ≥3 nuclei was counted. (E-F) IgG from patients with and without BD were treated with UDP-galactose to increase galactosylation and added to preosteoclasts for 2 to 4 days before the number of TRAP-positive cells with ≥3 nuclei was counted. The number of mature osteoclasts was evaluated by using TRAP stain. Data presented in panels A-F are relative to RANKL control. P values determined by Wilcoxon matched-pairs signed rank test. Symbols: purple diamond, sialic acid; yellow circle, galactose; blue square, GlcNAc; green circle, mannose; red triangle, fucose. ns, not significant.
Finally, we assessed if adding galactose to the immunoglobulins using β1-4 galactosyltransferase would influence osteoclastogenesis. Indeed, when immunoglobulins from patients with BD were treated with UDP-galactose and β1-4 galactosyltransferase, the pro-osteoclastogenic effect was significantly reduced (Figure 3F), whereas the same treatment had no effect in the no-BD group (Figure 3E). In conclusion, reduced galactosylation is mediating the pro-osteoclastogenic activity of the patient-derived immunoglobulins.
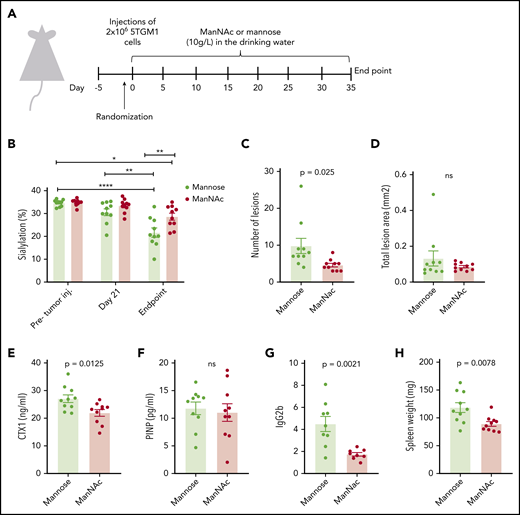
Modifying immunoglobulin glycosylation in vivo reduces tumor load and bone loss
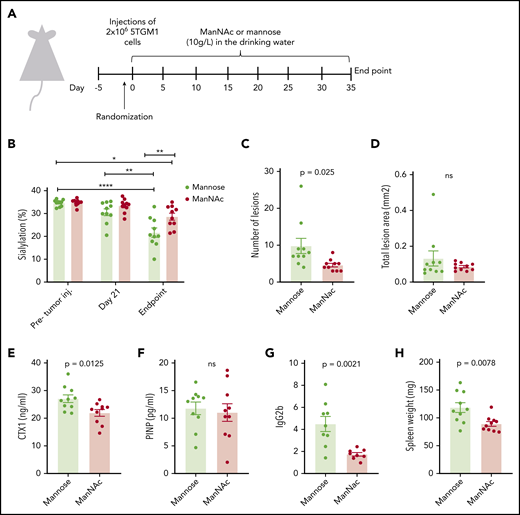
To investigate if modifying the extent of immunoglobulin glycosylation could have a beneficial effect on bone loss in vivo, we fed 5TGM1 myeloma mice ManNAc in the drinking water. ManNAc, a precursor for sialic acid, has previously been shown to increase immunoglobulin sialylation in mice.10 The experimental set-up is shown in Figure 4A. The 5TGM1 myeloma cells secrete IgG2b. As shown in Figure 4B, sialylation of the M-component (IgG2) decreased with disease progression. The reduction in sialylation was most prominent for the M component. There was a slight increase in Fc-sialylation of the uninvolved IgG1 (supplemental Figure 6A), whereas Fc-sialylation of IgG3 was reduced but to a lesser extent than for the M component (supplemental Figure 6B). Importantly, the ManNAc treatment significantly increased IgG2-Fc sialylation compared with the control mannose treatment. Moreover, a significant reduction was noted in the number of osteolytic lesions in the ManNAc-treated mice compared with the mannose-treated control group (Figure 4C). The lesions were larger (supplemental Figure 6G), and there was no difference in the total lesion area (Figure 4D). Importantly, however, a significant reduction was found in serum CTX-1, a degradation product of collagen type 1 (Figure 4E), indicative of reduced osteoclast activity. Together, these data support a reduction in osteoclast activity in the ManNAc-treated mice compared with the control mice. Serum levels of P1NP, a marker of osteoblast activity, did not differ between the groups (Figure 4F). Micro–computed tomography analysis showed that great variations occurred within the groups, and no significant differences in trabecular bone volume, trabecular thickness, trabecular separation, or trabecular number between the groups were found (supplemental Figure 6C-F). Of importance, ManNAc treatment reduced tumor load, as evident by reduced serum M component levels and reduced spleen weight in the ManNAc-treated mice compared with control mice (Figure 4G-H). The amount of plasma cells in the bone marrow also seemed reduced; however, this difference was not significant (supplemental Figure 6G).
Treatment with the sialic acid precursor ManNAc reduces the number of lesions and tumor load in vivo. (A) Twenty mice were given an intravenous injection of 2 × 106 of 5TGM1 mouse multiple myeloma cells and treated with ManNAc and the corresponding vehicle (mannose), both administered at 10 g/L in the drinking water for the duration of the study. After ∼30 days, the mice were euthanized. (B) IgG2abc-Fc sialylation before tumor challenge, 21 days’ post–tumor challenge, and at euthanasia as indicated. (C-D) The lesion number and lesion area in hind limbs were determined from radiographic images with MetaMorph image analysis software. (E-G) Serum levels of the osteoclast marker CTX-1, the osteoblast marker P1NP, and the M component IgG2b in animals treated as indicated were measured by enzyme-linked immunosorbent assay at the end of the study. (H) Spleens were harvested and weighed at end point. Significance was determined by 2-way ANOVA followed by Tukey’s multiple comparisons test (panel B) or by unpaired Student t test (panels C-H). *P < .05, **P < .01 ****P < .0001. ns, not significant.
Treatment with the sialic acid precursor ManNAc reduces the number of lesions and tumor load in vivo. (A) Twenty mice were given an intravenous injection of 2 × 106 of 5TGM1 mouse multiple myeloma cells and treated with ManNAc and the corresponding vehicle (mannose), both administered at 10 g/L in the drinking water for the duration of the study. After ∼30 days, the mice were euthanized. (B) IgG2abc-Fc sialylation before tumor challenge, 21 days’ post–tumor challenge, and at euthanasia as indicated. (C-D) The lesion number and lesion area in hind limbs were determined from radiographic images with MetaMorph image analysis software. (E-G) Serum levels of the osteoclast marker CTX-1, the osteoblast marker P1NP, and the M component IgG2b in animals treated as indicated were measured by enzyme-linked immunosorbent assay at the end of the study. (H) Spleens were harvested and weighed at end point. Significance was determined by 2-way ANOVA followed by Tukey’s multiple comparisons test (panel B) or by unpaired Student t test (panels C-H). *P < .05, **P < .01 ****P < .0001. ns, not significant.
Discussion
Most patients with multiple myeloma develop a severe osteolytic BD. The current understanding is that bone loss in multiple myeloma is multifactorial and that the main “driver” differs between patients.15 Accordingly, it is unclear why bone loss is such a common feature of this disease. The myeloma cells secrete immunoglobulins, and the presence of monoclonal immunoglobulins in the patients’ sera is an important measure for tumor load. Here, we show that immunoglobulins have a direct effect on bone loss in myeloma. Our data challenge the current conception and provide a new understanding of why patients with multiple myeloma lose bone.
We found that both sialylation and galactosylation of patient-derived M components are important for the effect on osteoclasts. We show that galactosylation is significantly different between patients with and without BD and that treating immunoglobulins from patients without BD with galactosidase to remove galactose makes the immunoglobulins pro-osteoclastogenic. When enzymatically adding galactose to the BD group–derived immunoglobulins, these immunoglobulins lost their pro-osteoclastogenic effect, confirming that galactose is important for the effect seen on osteoclasts. To investigate the effect of glycosylation in vivo, it is important to consider the differences between mice and men. In humans, galactosylation seems to have the largest influence on the inflammatory effects of immunoglobulins, whereas in mice, it is sialylation of the immunoglobulins that is most important.16,17 Interestingly, we found that IgG Fc sialylation is also reduced in mice upon disease progression. When we treated mice with a sialic acid precursor (ManNAc), the mice were to a certain extent protected from bone loss. The beneficial effect of ManNAc on bone, however, can also be explained by the unexpected reduction in tumor load we found in these mice. The effect of ManNAc treatment on tumor growth needs to be investigated further.
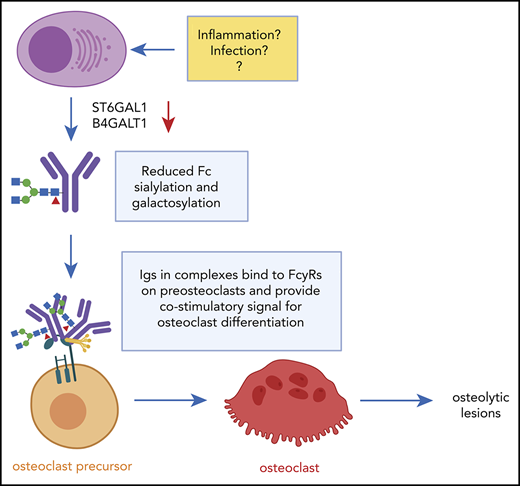
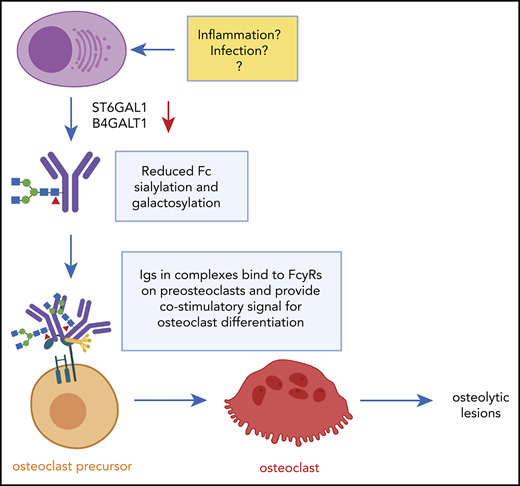
The “pro-osteoclastogenic” activity of immunoglobulins is specific for the M component. When treating preosteoclasts with uninvolved immunoglobulins (ie, isolating IgGs from patients with IgA myeloma), there was no effect on osteoclastogenesis in vitro. Reduced Fc-sialylation was reportedly a feature of both the M component and the uninvolved immunoglobulins in myeloma patients, although sialylation was significantly lower in the M component in individual patients compared with the uninvolved immunoglobulins.12 We observed a similar trend in mouse myeloma; the reduction in sialylation upon disease progression was most prominent for the M component, but there was a slight reduction in Fc sialylation also in the uninvolved immunoglobulins. Hence, it may be that there are other features in addition to reduced Fc-glycosylation that mediate the effect of the M component on osteoclasts. To this end, we report that the pro-osteoclastogenic effect is mediated by immunoglobulins in complexed or aggregated form. We cannot conclude if these are purely aggregates of misfolded immunoglobulins or if they consist of immunoglobulins bound to antigen or other proteins in “traditional” immune complexes. Studies have identified lysolipids,18,19 modified paratarg-7,20 and heat shock protein-9021 as antigens in subgroups of patients. We also found that the complement factors C1qrs bound more to immunoglobulins from patients with BD compared with those without BD, but no potential antigens were identified from the mass spectrometric analyses. However, this would be nearly impossible, as it is unlikely that the M component of these patients is targeted toward the same antigen. We propose that patients who develop BD have M components with reduced Fc-sialylation and galactosylation and a specificity for an endogenous or pathogen-derived antigen that lead to complex formation. These non-sialylated/non-galactosylated immune complexes promote osteoclast differentiation.
IgG Fc glycosylation is important for the immunoglobulin’s ability to mediate its effect, such as binding to Fcγ receptors, antibody-dependent cellular cytotoxicity activity, and aggregation behavior.22 During inflammation, serum IgGs are typically less sialylated and galactosylated.23 Conversely, in several cancers, increased sialylation of proteins are often common (as reviewed elsewhere24 ). In myeloma, results are conflicting. In one study, IgG sialylation was shown to be increased,25 and this increase correlated with disease stage. On the contrary, more recent studies have reported IgG sialylation to be decreased in myeloma patients.12,26 Here, we show that both IgG Fc sialylation and galactosylation are decreased in myeloma cases compared with healthy persons in a large cohort of well-characterized patients.
In conclusion, we have shown that immunoglobulins from myeloma patients with BD have reduced Fc-galactosylation and that these immunoglobulins induce osteoclastogenesis when present in the form of complexes or aggregates. Treatment with ManNAc to increase protein sialylation had a beneficial effect on both bone destruction and tumor load in a mouse model of myeloma. Taken together, our findings suggest that modifying immunoglobulin glycosylation may be beneficial for myeloma patients with bone loss.
All derived human IgG glycosylation traits are provided in supplemental Tables 2 and 3 available with the online version of this article. Other publication-related data will be made available by email to the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Berit Størdal, Hanne Hella, Helene Nilsen, and Jan Nouta for expert technical assistance. Patient materials were obtained from Biobank1.
This work was supported by funds from the Norwegian Cancer Society (#4500930), the Research Council of Norway (#223255 and #193072), the Liaison Committee for education, research and innovation in Central Norway (# 90061000 and # 90485500), the Cancer Fund at St. Olavs Hospital, and the National Natural Science Foundation of China (31901041).
Authorship
Contribution: M. Westhrin designed the study, performed experiments, analyzed data, and wrote the paper; V.K. performed experiments, analyzed data, and wrote the paper; Z.Z., S.H.M., T.M.V.N, A.B., S.H., K.M., G.B., T.S.S., A.W., A.S., and M. Wuhrer analyzed data and wrote the paper; and T.S. initiated and designed the study, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Therese Standal, Gastrosenteret 3 etg. sør, Prinsesse Kristinas gt 1, 7489 Trondheim, Norway; e-mail: therese.standal@ntnu.no.

![Glycosylation of immunoglobulins differs between myeloma patients with and without BD. (A) Heat map presenting glycosylation traits in the 3 groups: healthy control subjects (n = 51), no BD (n = 33), and BD (n = 43). TC, total complex; F, fucosylation; B, bisection; G, galactosylation; S, sialylation; S0, nonsialylated; F0, nonfucosylated; G0, nongalactosylated; A2, Diantennary glycans. The last letter indicates the subject of the calculation (eg, fucosylation [F]), and the preceding letters indicate the group on which it is calculated. Sialylation (B) and galactosylation (C) of immunoglobulins in healthy control subjects (n = 51), myeloma patients without BD (n = 33), and patients with BD (n = 43). *P < .05, **P < .01 using the Kruskal-Wallis test, then Dunnett’s multiple comparisons. Sialylation (D) and galactosylation (E) of individual patients before and after the onset of BD (n = 8). *P < .05 using Wilcoxon matched-pairs signed rank test. (F) Serum CTX-1 levels in patients with low (n = 17, lower 20th percentile) or high (upper 20th percentile) IgG galactosylation. (G) Volcano plot comparing glycosyltransferase expression in myeloma cells in patients without (n = 36) and with (n = 137) BD. Unadjusted P values are shown on the y-axis. Error bars represent mean ± standard error of the mean. *P < .05, **P < .01 using the Mann-Whitney U test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/136/23/10.1182_blood.2020006045/2/m_bloodbld2020006045f2.png?Expires=1767908778&Signature=pUGpteMEQHwVRqHPcD2k3LVr5Jq0I86G5-xy9ZnzIg~ndavYulve6uEJJJRIJ5EEGBpz7-P~IzMqgd00UB6KHLnxvZgByyf-vnQClN9Th1Q9rc9WhZgA7~yjQn-gXG-8JX5-nzQ8whXNgfMiqQA8isTajIGxBnKW6d8eDXYlsVK~tlIO7b9AOMIjbNbp-7-0wlYYi6d2lqSOEn5MzglrAbzo~juVwSZZ1mxMbokKu~~WoEa4~nCnJ2GhEOilKblji4Hn9QvRxWPK9aWfihvkflPXxGtuFlOo5k0CCZvew9bKvqf1QVIAU~jh10-6ZM4SCJcQJqkUTzOS063PiLlNeA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Glycosylation of immunoglobulins differs between myeloma patients with and without BD. (A) Heat map presenting glycosylation traits in the 3 groups: healthy control subjects (n = 51), no BD (n = 33), and BD (n = 43). TC, total complex; F, fucosylation; B, bisection; G, galactosylation; S, sialylation; S0, nonsialylated; F0, nonfucosylated; G0, nongalactosylated; A2, Diantennary glycans. The last letter indicates the subject of the calculation (eg, fucosylation [F]), and the preceding letters indicate the group on which it is calculated. Sialylation (B) and galactosylation (C) of immunoglobulins in healthy control subjects (n = 51), myeloma patients without BD (n = 33), and patients with BD (n = 43). *P < .05, **P < .01 using the Kruskal-Wallis test, then Dunnett’s multiple comparisons. Sialylation (D) and galactosylation (E) of individual patients before and after the onset of BD (n = 8). *P < .05 using Wilcoxon matched-pairs signed rank test. (F) Serum CTX-1 levels in patients with low (n = 17, lower 20th percentile) or high (upper 20th percentile) IgG galactosylation. (G) Volcano plot comparing glycosyltransferase expression in myeloma cells in patients without (n = 36) and with (n = 137) BD. Unadjusted P values are shown on the y-axis. Error bars represent mean ± standard error of the mean. *P < .05, **P < .01 using the Mann-Whitney U test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/136/23/10.1182_blood.2020006045/2/m_bloodbld2020006045f2.png?Expires=1767908779&Signature=rBRUHchYRViABA4h3PrA8XaJqqUROgu83bPS2JUGaLzrwdXmnPf7gCppPeQBM84Y57FO~SJUXTXWG6vPB-fhJfQ1QuGKZ4TZOaLE8e2JEcYAeUVoqyeZpTi~qpzjfm9ljnYqzTVC7i~MyL70BkQ0VLEpdQ~7NEiw8vZNclhyajX6cH8-sLAd4nTzlXZRELswy~TNhWAsdKLt9eE15KRL4mPOZG2KS3R4EdSJxev30UW~t0tePWjE-1oxPdE8Y8EaBBnEMI1vf5oc3bP2~qXFsFRndDScoboBe9QTIlrir9BoR82yxF9rxna8R~1Z6jbPS4mH9VD9-kqQEW89aWELOw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)