In this issue of Blood, Cooper et al show that a substantial proportion of adults with immune thrombocytopenia (ITP) presents asymptomatic cerebral microbleeds (CMBs) as revealed by susceptibility-weighted magnetic resonance imaging (SWI), as illustrated in the figure. This unexpected finding raises critical questions both for the individual patient and for the management strategy of ITP.1
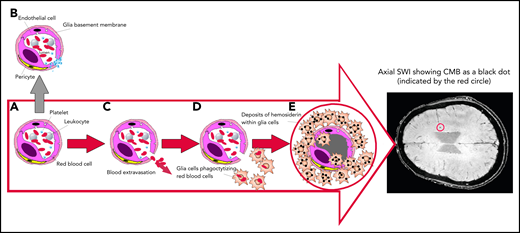
Mechanism of SWI of occult CMBs. Breaks in the exceedingly small blood vessels, like capillaries or postcapillary venules, are followed by extravasation of a thin amount of blood. The wall of these microvessels, made by interconnected endothelial cells, covered by a glia basement membrane with embedded pericytes (A), prevents blood from leaking out. If vessel damage occurs in healthy people, a multilayer of platelets is immediately formed to close the break, avoiding blood extravasation (B). However, if platelets are severely reduced, like in ITP, blood cells can escape and invade the cerebral parenchyma (C), inducing glia cells activation into macrophages that phagocytize escaped cells and degrade the hemoglobin into nontoxic hemosiderin deposits (D), preventing the toxicity of free iron. Hemosiderin-laden macrophages (E) may persist indefinitely. Because of their high iron content, SWI allows the detection of CMBs, that appear as small black dots, as shown in the encircled area of the axial brain representation.
Mechanism of SWI of occult CMBs. Breaks in the exceedingly small blood vessels, like capillaries or postcapillary venules, are followed by extravasation of a thin amount of blood. The wall of these microvessels, made by interconnected endothelial cells, covered by a glia basement membrane with embedded pericytes (A), prevents blood from leaking out. If vessel damage occurs in healthy people, a multilayer of platelets is immediately formed to close the break, avoiding blood extravasation (B). However, if platelets are severely reduced, like in ITP, blood cells can escape and invade the cerebral parenchyma (C), inducing glia cells activation into macrophages that phagocytize escaped cells and degrade the hemoglobin into nontoxic hemosiderin deposits (D), preventing the toxicity of free iron. Hemosiderin-laden macrophages (E) may persist indefinitely. Because of their high iron content, SWI allows the detection of CMBs, that appear as small black dots, as shown in the encircled area of the axial brain representation.
ITP is the most frequently acquired isolated thrombocytopenia affecting children and adults, with an annual incidence of 3 to 6 new cases per 100 000. ITP is caused by autoantibodies and autoreactive lymphocytes that recognize megakaryocytes and platelets, leading to insufficient production of platelets to compensate for their increased destruction. The diagnosis is still by exclusion, requiring a platelet count <100 × 109/L without apparent cause.
Bleeding, particularly in skin and mucosae, is limited to patients with a platelet count <50 to 30 × 109/L. In children, most cases resolve spontaneously and are usually untreated due to the lower risk of bleeding. By contrast, in ∼70% of adults, the disease is chronic, and treatment is often required to permit a near-normal or normal lifestyle by reducing the risk of major hemorrhage. In most patients, significant bleeding is rare unless the platelet count is <20 × 109/L and may be absent or minimal even with a count as low as 5 to 10 × 109/L. In other patients with similar counts, bleeding may be impressive, particularly at diagnosis, with extensive purpura and mucosal hemorrhage. The overall bleeding mortality rate in adults is estimated to be ∼1% to 2%. However, the risk is much higher, up to 10% to 15%, in older unresponsive patients. The weak and intriguing relationship between bleeding severity and platelet count has not been fully explored. From 1 side, more young and active platelets may circulate in some phases of the disease, explaining the patient’s shorter bleeding time at the same platelet count compared with other thrombocytopenic statuses.2 On the other side, the endothelium is also a critical component, and its fragility in ITP was implied by older studies showing that corticosteroids improved skin and mucosal bleeding manifestations 1 to 2 days before any platelet count increase was observed.3,4 Finally, we now know from immunopathology studies that ITP patients have an increased concentration of proinflammatory cytokines.5 These 3 factors, namely platelet intrinsic activity, endothelium damage, and inflammation, contribute to the unpredictability of the bleeding risk and to the weak correlation with the actual platelet count. These uncertainties cause anxiety in patients (and doctors). Accordingly, the main goal of treatment in adults is to raise platelet count to an ill-defined minimal level, often referred to as a “safe platelet count,” thought to be protective. Paradoxically, a slightly increased risk of arterial and venous thrombosis coexists in ITP and may be increased by some treatments.6 Unfortunately, for most cases, curative approaches are not yet available, and despite the current wide options of treatments, none is without significant adverse effects.
The findings of Cooper et al further underscore the complexity of ITP. Moving from the poor predictability of ITP severity and recognizing that current treatment guidelines, still based on platelet count, are confounded by variable bleeding phenotypes, the authors hypothesized that imaging the brain for CMBs with SWI could provide a sensitive noninvasive biomarker of occult hemorrhage. This could help the identification of patients with more hemorrhagic phenotypes and improve future stratification of treatment.
Forty-nine adult ITP patients who had at least 1 lowest recorded platelet count <30 × 109/L (nadir) during the course of their disease and 18 normal controls (both groups with mean age between 40 and 45 years) were investigated using SWI of the brain. In comparison with the less-sensitive widely used gradient echo T2* MRI, Cooper et al adopted an improved technique generating additional tissue contrast. CMBs were identified in 43% of patients (21/49) with prevalence increased with decreasing nadir platelet count. The absence of CMBs in patients with a platelet nadir >15 × 109/L and in all 18 healthy controls clearly substantiates ITP as the causative or permissive factor for CMBs. Statistically significant associations of total CMBs per subject were found with longer disease duration, initiated during childhood in some cases, possibly indicating that these microhemorrhages accumulate over time, particularly in refractory cases. Prevalence of CMBs was also associated with lower platelet counts at the time of SWI and with higher organ bleeding scores, but not with mucosal and skin bleeding scores or with the number of previous treatments, age, and sex. In summarizing their findings, Cooper et al suggest that applying a treatment threshold based on platelet count alone could result in some patients being overtreated and others undertreated, and that SWI could provide a specific noninvasive biomarker of central nervous system hemorrhagic tendency, enabling further stratification of disease phenotypes. The inability to link the occurrence of a CMB to a specific time during the course of the disease is a major limitation of this study. Also, as suggested by the authors, longitudinal prospective studies are needed, in particular, to prove that there are increasing numbers of CMBs over time. In a similar study limited to children and adolescents, only 1 case with a single CMB was found among 27 prospectively investigated subjects, all with a platelet count ≤10 × 109/L at diagnosis or upon symptomatic relapse.7 Reassuringly, in patients with severe hemophilia, a much more harmful hemorrhagic disease, CMBs were only slightly increased compared with healthy controls.8
Appropriately, Cooper et al do not recommend brain SWI outside of a research setting. Minor cognitive symptoms with memory and concentration difficulties have been reported in some patients with ITP, but they were ascribed to emotional distress and often associated with other common subjective symptoms like fatigue9 Cautiously, the authors avoid making conjectures on the possible ominous long-term neurological consequences of CMBs in ITP.
This excellent study raises more issues than it resolves. It will encourage new areas of investigation to clarify the significance of CMBs in ITP and to better understand the mechanisms underlying different bleeding phenotypes beyond the simplistic parameter of the platelet count.
Conflict-of-interest disclosure: The author declares no competing financial interests.