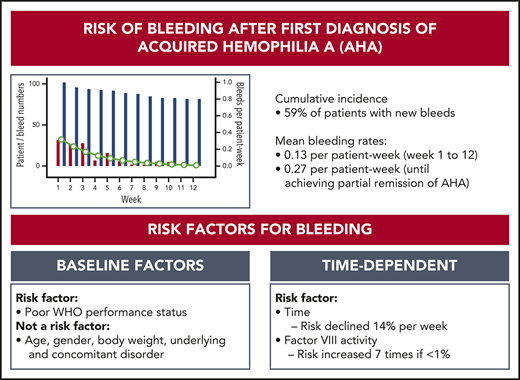
Key Points
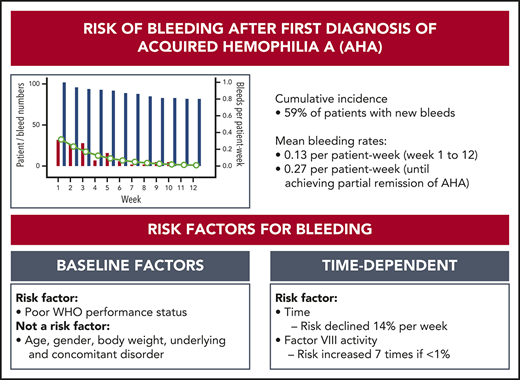
Patients with AHA have a high risk of recurrent bleeding until they achieve partial remission of their disease.
Residual FVIII activity and clinical performance status are associated with recurrent bleeding.
Abstract
Acquired hemophilia A (AHA) is due to autoantibodies against coagulation factor VIII (FVIII) and most often presents with unexpected bleeding. In contrast to congenital hemophilia, the patient’s residual FVIII activity does not seem to correlate with the risk of bleeding as suggested from previous studies. Risk factors for bleeding have not been described. We used data from the prospective GTH-AH 01/2010 study to assess the risk of bleeding and the efficacy of hemostatic therapy. FVIII activity was measured at baseline and weekly thereafter. Bleeding events were assessed by treating physicians. A total of 289 bleeds were recorded in 102 patients. There were 141 new bleeds observed starting after day 1 in 59% of the patients, with a mean rate of 0.13 bleed per patient-week in weeks 1 to 12, or 0.27 bleed per patient-week before achieving partial remission. Weekly measured FVIII activity was significantly associated with the bleeding rate, but only achieving FVIII activity ≥50% abolished the risk of bleeding. A good World Health Organization performance status assessed at baseline (score 0 vs higher) was associated with a lower bleeding rate. Hemostatic treatment was reportedly effective in 96% of bleeds. Thus, the risk of new bleeds after a first diagnosis of AHA remains high until partial remission is achieved, and weekly measured FVIII activity may aid in assessing the individual risk of bleeding. These results will help to define future strategies for prophylaxis of bleeding in AHA.
Introduction
Acquired hemophilia A (AHA) is an autoimmune disorder characterized by bleeding due to neutralizing antibodies against coagulation factor VIII (FVIII).1-3 Bleeds most often occur into soft tissues, including muscles and skin, but also in the gastrointestinal and urogenital tracts.4,5 Bleeds can be severe or even life-threatening and are often difficult to treat.6-8
Immunosuppressive therapy (IST) is used to suppress formation of anti-FVIII autoantibodies and results in remission of AHA after several weeks or months.4,9-12 Remission can also occur spontaneously without IST, but the time needed to achieve spontaneous remission seems to be very long.13,14 The high risk of bleeding and the significant bleed-related morbidity resulted in recommendations to administer IST to all patients with AHA irrespective of their bleeding phenotype.1,15,16 However, IST has significant side effects in patients with AHA. In particular, the risk of dying from infection currently seems to be higher than the risk of fatal bleeds.4,5,10,11
Not much is known, in fact, about the magnitude of the bleeding risk in AHA. In the European Acquired Hemophilia Registry (EACH2), the largest available data set, bleeding was reported as the trigger for diagnosis in 89% of patients.5 FVIII activity or anti-FVIII inhibitor concentration assessed by local laboratories at the time of diagnosis did not seem to be related to the risk of bleeding, severity of bleeds, or response to hemostatic treatment. However, EACH2 confined its analysis to the patient’s first bleed; the risk of subsequent bleeds is basically unknown.
We believed it would be helpful to address the risk of bleeding in AHA more carefully. Clinically useful predictors of bleeding could be used to better tailor IST to individual patients and to address the potential need for a prophylactic hemostatic treatment in patients with AHA. We used data from the GTH-AH 01/2010 registry, a multicenter prospective observational study of patients with AHA.10 The primary objective of this registry was to study prognostic factors for survival and for achieving partial remission (PR) and complete remission (CR) with a consensus IST protocol.3,17 However, this study also collected information on bleeds and hemostatic therapy, which is reported here.
Methods
Study design and population
The GTH-AH 2010 study, a prospective observational study in patients with AHA, was conducted in 29 centers in Germany and Austria between 2010 and 2013. Patients were enrolled consecutively and treated with IST immediately after diagnosis, following a GTH consensus protocol. Diagnosis of AHA was confirmed by the presence of a FVIII inhibitor of >0.6 Bethesda units (BU) and FVIII activity <50%. Patients were enrolled in the registry within 7 days of starting steroids. Details of the study protocol and the primary analysis have been reported previously.10 The treatment of bleeds was at the discretion of the local investigator. The research protocol was approved by the ethics committees of participating institutions, and the research was conducted in accordance with the Declaration of Helsinki.
Clinical data and end points
For each bleeding event, time of onset, duration, location, severity, treatment, and outcome were reported by the treating physician in a standardized case report form. All clinically relevant bleeding events were recorded, regardless of severity, treatment requirement, and recurrence status, and were reported as “total bleeds.” Severe bleeds were defined as fulfilling at least one of the following criteria: life-threatening, leading to organ failure or compartment syndrome, hemoglobin <8 g/dL or a drop by >2 g/dL per 24 hours, and requiring transfusion of >2 units of red blood cells per 24 hours. Treated bleeds were defined as those that prompted a new or more intense therapy with bypassing agents or FVIII concentrates. Recurrent bleeds were defined as those occurring in an anatomic site where the patient had a previous, already controlled bleed (regardless of the time interval between the initial bleed and the recurrent bleed); nonrecurrent bleeds were defined as those occurring in an anatomic site where the patient had not had a bleed previously. The time point of bleeding cessation was assessed by the treating physician according to clinical findings and/or imaging and stable hemoglobin levels. Bleeding treatment was guided by the local physician and recorded on a patient level, including dose, interval, duration, and efficacy of treatment with bypassing agents, human FVIII (hFVIII) concentrates, tranexamic acid, other hemostatic agents, or blood products. Clinical data and FVIII activity were collected once weekly until CR or death. FVIII activity was categorized into 5 clinically meaningful intervals: <1%, 1% to <5%, 5% to <20%, 20% to <50%, and ≥50%. FVIII activity was measured in local laboratories by using one-stage clotting assays. Bethesda inhibitor titer was determined at baseline according to local standard procedures. The definitions used for PR and CR were reported previously.10
Statistical analysis
For consistency with previous GTH study reports, day 1 was defined as the day of starting steroid therapy. Week 1 was defined as starting the day after day 1. The basic analysis and treatment analysis were performed by using GraphPad Prism 8.0. Means and standard deviations are presented for numeric variables, and absolute and relative frequencies are presented for categorical variables. Numbers of patients receiving different hemostatic drugs were compared by using χ2 tests. Doses were compared by using Mann-Whitney U tests or Kruskal-Wallis tests where appropriate. For the bleeding risk analysis, bleeds occurring week 1 to 12 were considered. Time to a first new bleed was analyzed by using the Kaplan-Meier method. The mean rate of bleeds per patient-week was estimated by using the negative binomial model. For evaluating the impact of various baseline variables and current weekly FVIII activity on the number of weekly bleeds, negative binomial log-linear mixed models were applied using the GLIMMIX procedure in SAS version 9.4; maximum likelihood with adaptive quadrature was specified as the estimation method and patient was treated as a random effect. A univariate analysis, adjusted only for the observation week, was first performed for each independent variable. Variables with statistically significant impact in the univariate analysis were then included in a multivariate model for the joint assessment of their impact on the bleeding risk. Rate ratios and their 95% confidence intervals were presented. For all analyses, a P value <.05 was considered statistically significant.
Results
A total of 102 patients with AHA were enrolled in the study from 19 of 29 participating centers in Austria and Germany. Demographic characteristics, baseline characteristics, and numbers of bleeding events are reported in Table 1. Ninety-three (91%) of the 102 patients had at least 1 bleed documented, and a total of 289 bleeds (2.8 bleeds per patient) were documented in these patients. About one-half of all bleeds occurred before day 1 (n = 148) and from day 1 onward (n = 141).
Basic analysis of bleeds occurring before and after day 1
Table 2 reports raw numbers of bleeds according to time of occurrence, severity, and location. Bleeds starting before day 1 were more often severe (51%) compared with bleeds starting after day 1 (33%; P = .002). The most frequent bleeding locations were muscles (43% of all bleeds), skin (30%), and the gastrointestinal tract (9%). Gastrointestinal bleeds were the only type of bleed that occurred more often after day 1 (89%) than before day 1 (11%). Bleeds occurring before day 1 were significantly longer compared with bleeds occurring after day 1. There was no difference in duration when comparing severe and nonsevere bleeds separately for time of occurrence. Fatal bleeds occurred in 3 patients (2.9%). Details on these bleeds are provided in supplemental Table 1 (available on the Blood Web site).
Bleeding risk analysis
Cumulative bleeding risk
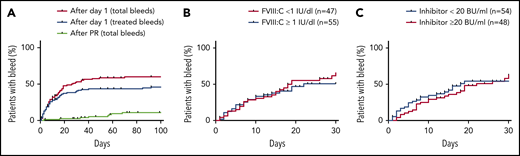
We first analyzed the cumulative incidence of total bleeding events and treated bleeding events after day 1 (Figure 1). Sixty-one (59%) of the 102 patients had at least 1 bleeding event, and 48 (47%) had at least 1 treated bleeding event. The incidence of bleeding events was very low after achieving the first PR, indicating a protective effect of FVIII activity ≥50%. Baseline FVIII activity and Bethesda inhibitor titer did not influence the cumulative incidence of new bleeds after first diagnosis.
Kaplan-Meier analysis of bleeding risk according to baseline characteristics and remission status. (A) Frequency of patients with bleeding event after day 1 of study (102 patients; red line, total bleeds; blue line, treated bleeds only) and after the day of first PR (81 patients; green line, total bleeds). All events include recurrent and nonrecurrent bleeding events. (B) Frequency of patients with bleeding event after day 1 according to baseline FVIII activity (102 patients). (C) Frequency of patients with bleeding event after day 1 according to baseline FVIII inhibitor (102 patients). In all panels, patients no longer at risk (because of death or end of follow-up) were censored.
Kaplan-Meier analysis of bleeding risk according to baseline characteristics and remission status. (A) Frequency of patients with bleeding event after day 1 of study (102 patients; red line, total bleeds; blue line, treated bleeds only) and after the day of first PR (81 patients; green line, total bleeds). All events include recurrent and nonrecurrent bleeding events. (B) Frequency of patients with bleeding event after day 1 according to baseline FVIII activity (102 patients). (C) Frequency of patients with bleeding event after day 1 according to baseline FVIII inhibitor (102 patients). In all panels, patients no longer at risk (because of death or end of follow-up) were censored.
Bleeding rates
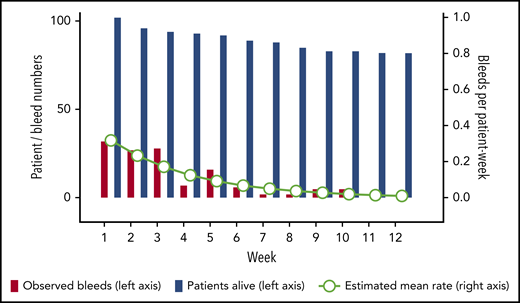
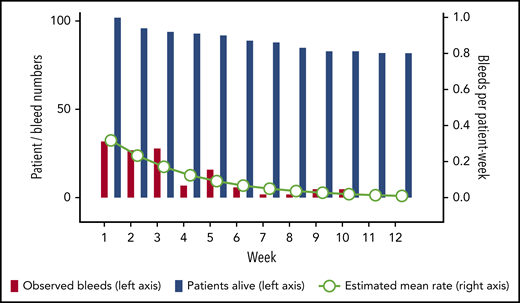
A total of 130 (92%) of the 141 bleeds occurring after day 1 started within the first 12 weeks. Thirty events (24% of 127 events with sufficient information) represented recurrent bleeds into previously affected sites, whereas the remaining 97 events (76%) were assessed as truly new (nonrecurrent) bleeds. Mean bleeding rates per patient-week in weeks 1 to 12 were estimated according to a negative binominal distribution function. Figure 2 illustrates good agreement between the observed number of bleeds and the model-estimated weekly bleeding rate. The mean rate of total bleeding events in weeks 1 to 12 was 0.13 event per patient-week (95% confidence interval, 0.10-0.17). Confining the analysis to the time before achieving PR (or until week 12, whichever occurred first), the mean rate of total bleeding events was 0.27 per patient-week (95% confidence interval, 0.21-0.34). Rates of total, treated vs untreated, and recurrent vs new bleeds are summarized in Table 3.
Rate of total bleeds in weeks 1 to 12. The bars show crude numbers of observed bleeds (red bars) in patients alive (blue bars) according to treatment week (left y-axis). Circles show the mean estimated weekly bleeding rate according to negative binomial distribution for patients alive (right y-axis).
Rate of total bleeds in weeks 1 to 12. The bars show crude numbers of observed bleeds (red bars) in patients alive (blue bars) according to treatment week (left y-axis). Circles show the mean estimated weekly bleeding rate according to negative binomial distribution for patients alive (right y-axis).
Prognostic factors for bleeding
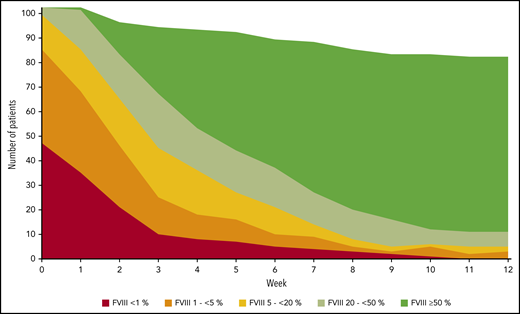
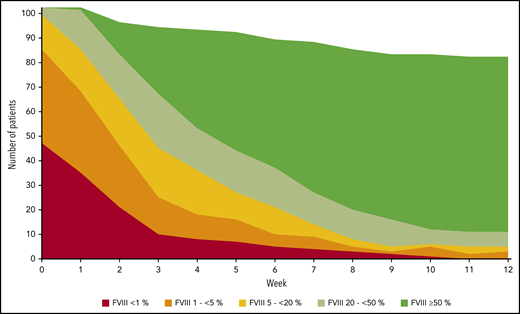
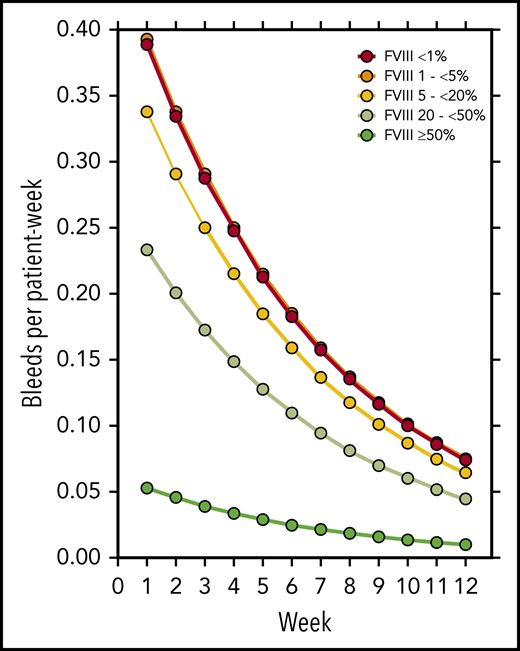
FVIII activity was collected throughout the study every week, at least until PR was achieved. Figure 3 illustrates the number of patients in each of the five FVIII activity categories over time. Negative binomial log-linear mixed models were applied to evaluate the impact of the current, weekly FVIII activity category and patient-related baseline characteristics on the bleeding rate (Table 4). The influence of FVIII activity was highly statistically significant; World Health Organization (WHO) performance status, heart failure, renal failure, and diabetes mellitus had a modest influence in univariate models. Entering these factors into a multivariate model, only the FVIII activity and WHO performance status were predictors of the weekly bleeding rate. The model-based predicted effect of time and FVIII activity is shown in Figure 4. Similar results were obtained when the analysis was confined to treated bleeds (supplemental Table 2).
FVIII activity over time. FVIII activity was assessed at least weekly by local laboratories. Filled areas show the absolute number of patients in each FVIII activity category over time. Week 0 refers to the last 7 days before day 1. If more than one FVIII activity was available in a week, the lowest activity was used for analysis.
FVIII activity over time. FVIII activity was assessed at least weekly by local laboratories. Filled areas show the absolute number of patients in each FVIII activity category over time. Week 0 refers to the last 7 days before day 1. If more than one FVIII activity was available in a week, the lowest activity was used for analysis.
Model-estimated bleeding rate according to treatment week and current FVIII activity.
Model-estimated bleeding rate according to treatment week and current FVIII activity.
Treatment analysis: bleed level
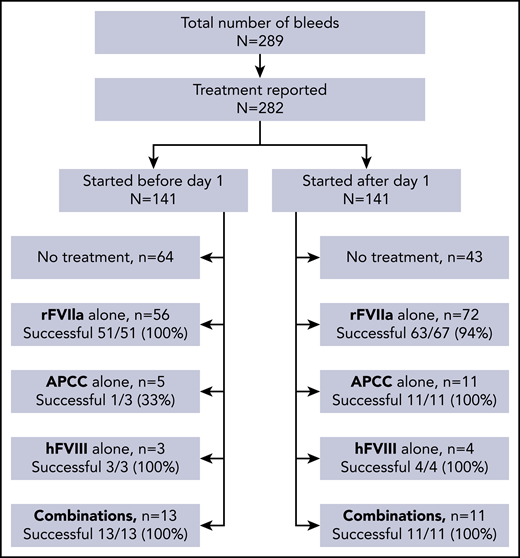
Treatment information was available for 282 of 289 bleeds, including 119 severe bleeds and 162 nonsevere bleeds. Hemostatic treatments as chosen by local investigators and treatment success are given in Figure 5. Bleeds starting before day 1 more often remained untreated (64 of 141 bleeds [45%]) than bleeds starting after day 1 (43 of 141 [30%]). Treatment success of recombinant factor VIIa (rFVIIa), activated prothrombin complex concentrate (APCC), hFVIII, and combinations thereof was generally high and did not differ for bleeds starting before and after day 1. There was also no difference in the efficacy of treatment of severe and nonsevere bleeds (not shown).
Dosing information for hemostatic agents was not recorded on the bleed level but on the patient level in the GTH registry (see "Treatment analysis: patient level"). Detailed information for 48 bleeds, to which rFVIIa dosing information could be unambiguously assigned, is provided in supplemental Table 3. Starting doses were slightly lower for nonsevere bleeds compared with severe bleeds. More pronounced, however, were differences in the initial dosing interval, with nonsevere bleeds treated more often with single doses or long dosing intervals compared with severe bleeds.
Treatment analysis: patient level
Hemostatic treatment information and blood product use were collected on the patient level. Adverse events, including thromboembolic events, have been previously reported.10 No treatment was given in 21% of patients, and no bypassing agents were required in 33% of patients (Table 5). Treatment duration and cumulative doses of rFVIIa (given to 62% of patients), APCC (22%), and hFVIII (15%) were highly variable. Tranexamic acid was administered in 30% of patients, including 33% of patients treated with rFVIIa and 27% with APCC. Tranexamic acid was administered for a median of 15 days with an average of 2 g/d. Packed red blood cells were required in 46% of patients; other blood products, including platelet concentrates, fresh frozen plasma, and fibrinogen concentrate, were used in only a few patients. Detailed information on all hemostatic treatments and blood products, including cumulative treatment doses, body weight, and bleed numbers, are provided in supplemental Table 4.
Discussion
The current article describes bleeding events in a prospective cohort of patients with AHA. Bleeding symptoms and other clinical characteristics were collected weekly and were available in all patients at least until they achieved PR or died. Baseline clinical and laboratory characteristics of the current study patients were similar to those from previous registries of AHA.5,11,13,18,19 However, most previous registries focused on describing the bleeding phenotype at presentation or each patient’s first bleed. Our study is unique in assessing the overall bleeding risk, including the quantitative risk of new bleeds after diagnosis, in an unselected prospective cohort. We found an average of 2.8 bleeds per patient, with one-half of these bleeds occurring before day 1. Bleeds occurring before day 1 were more severe and lasted longer than bleeds occurring after day 1. This observation is likely to reflect delays in diagnosis of AHA and underlines again the importance of awareness toward this rare disorder.
An important outcome of our study is the description of the risk of new bleeds after diagnosis, including “recurrent” and “nonrecurrent" (truly new) events, which occurred altogether in 59% of patients. There was a statistically significant trend toward lower bleeding rates with increasing FVIII activity, but only achieving PR (defined as FVIII ≥50%) protected patients substantially from bleeding. These data are consistent with the clinical experience that residual FVIII activity in AHA does not entirely protect from bleeding. It is in sharp contrast to congenital hemophilia, in which spontaneous bleeds are very rare when FVIII is >12% to 15%.20 Our patients with AHA had a fourfold to fivefold higher risk of bleeding while at 20% to 50% FVIII activity compared with >50%. Altogether, these results support the concept of eliminating the inhibitor and restoring FVIII activity by IST, because only achieving PR seems to substantially protect patients from bleeding. IST is associated with a high risk of infectious complications in patients with AHA, however, and more deaths were attributed to adverse effects of IST than to bleeding, as noted in other publications.4,5,11 This dilemma highlights the lack of safe and effective hemostatic prophylaxis as an unmet medical need in AHA.
Of note, there was a decrease in the bleeding tendency over time that was independent of FVIII activity level (Figure 4). The remarkably high bleeding risk early after diagnosis may be attributable to: (1) the risk of recurrent bleeds into previously affected bleeding sites (accounting for ∼24% of all bleeding events after day 1); (2) local risk factors for bleeding (eg, gastric ulcers); (3) interventions (eg, catheter placement or surgery); or (4) minor trauma in patients with physical or mental impairment. Over time, these potential risk factors may resolve to at least some extent, resulting in a decline of the bleeding risk. A good WHO performance status at baseline also seemed to protect from bleeds, indicating that less ill patients may have fewer of the risk factors noted earlier. In addition, we hypothesize that hemostatic agents (bypassing agents, tranexamic acid) given longer than just for the resolution of a previous bleed in patients with an apparently high risk of bleed recurrence may have had protective effects.
The current study found no influence of underlying disorders (eg, malignancy, autoimmunity, peripartum period) on the risk of bleeding after the initial diagnosis of AHA. Malignancy as a potential risk factor for AHA and bleeding has been discussed recently.21 However, the number of patients with malignancy was too small in our cohort to analyze the impact of cancer type and activity status on clinical outcomes of AHA.
Our key findings should be discussed in light of previous registries in AHA. The UK surveillance study found that baseline FVIII activity and inhibitor titer were not predictive of severity of bleeding.4 Median FVIII:C in patients with fatal bleeding (13 of 143 [9%]) and in those who did not require hemostatic therapy (51 of 149 [34%]) was 4 IU/dL (range, <1 to 12 IU/dL) and 3 IU/dL (range, <1 to 25 IU/dL), respectively. Similar observations were made for the Bethesda titer. In the French SACHA (Surveillance des Auto antiCorps au cours de l’Hémophilie Acquise) registry,11 patients with >1 bleeding site or life-threatening bleeds received hemostatic therapy more often than other patients but did not differ with regard to baseline FVIII activity or inhibitor titer. Of note, the association between baseline FVIII activity or inhibitor titer and the bleeding risk was also poor in our study (Figure 1 B-C). However, FVIII activity over time, which was significantly correlated with the bleeding rate, was much more informative.
SACHA reported recurrent bleeding in 26 (32%) of 82 patients.11 The rate observed in our study (59%) was somewhat higher, possibly due to the fact that the bleeding status had to be actively assessed by our participating physicians every week. The EACH2 registry focused its analysis on first bleeds and their first-line hemostatic therapy only.5,22 As such, bleeds occurring after a diagnosis of AHA were recorded infrequently (<5% of patients). The total number of bleeds per patient and the bleeding risk over time were not reported from this registry. The Hemostasis and Thrombosis Research Society Registry19 was initially designed as a surveillance of rFVIIa therapy in the United States. Overall, 237 bleeding episodes were recorded in 110 patients with AHA. This frequency of 2.2 bleeds per patient was similar to our study, but information on the bleeding risk over time or its association with FVIII activity was not reported. Other registries either did not report information on bleeds or did not relate FVIII activity to bleeding risk.13,18
Taken together, our prospective study confirms most data from previous registries but adds a new perspective in describing the bleeding risk over time and the impact of residual FVIII activity.
Our study also collected information on hemostatic treatments and their efficacy. Most bleeds were treated with rFVIIa and less frequently with APCC, hFVIII, or combinations of these drugs. Consumption data, success rates, and adverse events were consistent with previous literature.19,23-28 The frequent use of tranexamic acid in 30% of patients is unique in our study and further adds to the safety record of this drug in a population of acutely ill, mostly elderly patients. We did not report data on recombinant porcine FVIII because it was not yet available at the time of patient enrollment.
Several limitations of our study should be noted. The exact starting day of bleeds occurring before day 1 was sometimes unknown, preventing the calculation bleeding rates for this period. FVIII and inhibitor levels as reported here were measured by local laboratories according to our protocol.10 Physicians should be aware of the high interlaboratory variability of coagulation factor assays29 when integrating our findings into their clinical practice. Conversely, this situation may reflect the real-world situation better than data obtained from a single center or a central laboratory.
We were unable to report hemostatic treatment doses and treatment durations on the bleed level (except for 48 bleeds) because the data were primarily acquired on the patient level. When designing the GTH study, it was considered complicated to collect treatment information on the bleed level because 1 treatment may be given for >1 bleed at a time. However, we recognize this lack of data as a limitation today and suggest that future registries should record hemostatic therapy on both the patient and the bleed level.
In summary, our study is the first, to our knowledge, to systematically assess the bleeding risk in AHA over time in the framework of a prospective observational study. We found that weekly measurement of FVIII activity helps to assess the individual bleeding risk, and we show for the first time the high incidence of recurrent bleeds after day 1, accumulating to ∼60% of the patients. These results will be helpful in defining the need for prophylactic hemostatic therapy for AHA in the future.
All requests for original data should be submitted to the corresponding author via e-mail.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was conducted by the German, Austrian, and Swiss Society on Thrombosis and Haemostasis (GTH e.V.). Contributing investigators are listed in the supplemental Appendix. The authors acknowledge the contribution of all study sites, local study coordinators, and Susanne Bartels, central study coordinator at Hannover Medical School. Armin Koch, Director of the Institute of Biometry at Hannover Medical School, is acknowledged for kindly supporting and supervising the statistical analysis.
Funding for this study was obtained from GTH e.V. and by unrestricted educational grants from Novo Nordisk Pharma and Shire/Takeda (previously Baxalta).
Authorship
Contribution: A.T. designed the study; A.T. and K.H. interpreted the data and wrote the manuscript; X.L., A.S., and A.T. performed the statistical analysis; P.K., R.K., U.G., W.M., and H.E. enrolled patients, collected and reviewed clinical data, and contributed to manuscript writing; and all authors critically reviewed the manuscript and approved of its publication.
Conflict-of-interest disclosure: K.H. received honoraria for advisory boards or speaker fees from Bayer, Biotest, Chugai, CSL Behring, Novo Nordisk, Pfizer, Roche, Shire/Takeda, and SOBI; and unrestricted research grants from Bayer, CSL Behring, and Pfizer. P.K. received honoraria, consultation and speaker fees, and research and travel grants from Biotest, CSL Behring, Novo Nordisk, Octapharma, Roche, Sanofi, and Baxalta/Shire/Takeda. R.K. received research funding and honoraria from Bayer, BioMarin, Biotest, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, SOBI, and Takeda. U.G. received honoraria for advisory boards and travel grants from Roche, Bayer, and Baxalta/Shire/Takeda. H.E. received honoraria or consultation fees, advisory boards or speaker fees, and research grants from Bayer, Biotest, CSL Behring, Novo Nordisk, Pfizer, Roche, Shire, and SOBI. W.M. received honoraria, research grants, and consultation fees for participating at educational meetings organized by Alnylam, Bayer, Biogen Idec, Biotest, Chugai, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Shire, and SOBI. A.T. received honoraria or consultation fees for participating at educational meetings organized by Alnylam, Bayer, Biogen Idec, Biotest, Boehringer Ingelheim, Chugai, CSL Behring, Daiichi Sankyo, Leo Pharma, Novo Nordisk, Octapharma, Pfizer, Portola, Roche, Shire, and SOBI. The remaining authors declare no competing financial interests.
Correspondence: Andreas Tiede, Hannover Medical School, Department of Hematology, Hemostasis, Oncology and Stem Cell Transplantation, Carl Neuberg Str. 1, 30625 Hannover, Germany; e-mail: tiede.andreas@mh-hannover.de.