Background: FXIa inhibition is a promising antithrombotic drug target. BMS-986177/JNJ-70033093 (BMS-177/JNJ-3093) is a novel small molecular inhibitor of FXIa currently in Phase II clinical trials with the potential for reduced bleeding risk as compared to the currently approved oral anticoagulantsHowever, reversal of anticoagulation may still be required in patients who have uncontrolled or life-threatening bleeding or need an urgent surgical procedure.
Aim: To evaluate the ability of nonspecific reversal agents (NSRAs) FEIBA®, NovoSeven®, Kcentra®, Profilnine®, BeneFix®, Novoeight®, and Cyklokapron® to neutralize the anticoagulation of BMS-177/JNJ-3093 in the activated partial thromboplastin time (aPTT), thromboelastography (TEG) and thrombin generation assay (TGA) in vitro using human plasma or whole blood.
Method: aPTT and TEG were performed in human plasma and whole blood, respectively, using standard assay procedures. TGA was performed in human plasma using diluted kaolin aPTT reagent (1:10,000). JNJ-3093 was evaluated at different concentrations (0.3 -10 µM) to cover the anticipated exposures in the Phase II clinical trials. The NSRAs were evaluated at the anticipated concentrations according to the dosing information in their respective labels.
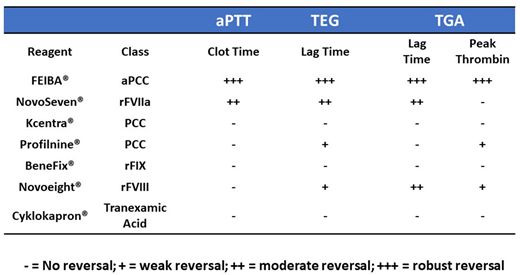
Results: BMS-177/JNJ-3093 produced concentration dependent increases in aPTT (up to 4.4x at 10 μM); prolongations of lag time in TEG (2.6X); prolongations of lag time (3X) as well as reductions in peak thrombin generation (70%) in TGA. FEIBA® effectively neutralized the anticoagulant effects of JNJ-3093 in aPTT, TEG and TGA. NovoSeven® neutralized the BMS-177/JNJ-3093-induced prolongations in aPTT, prolongations in lag time in TEG and TGA assays and partially restored the peak thrombin generation in TGA. In contrast, all other NSRAs tested had negligible effects or did not show neutralization of anticoagulation induced by BMS-177/JNJ-3093 in the referenced assays
Conclusion: These results demonstrate that FEIBA® and NovoSeven® can effectively neutralize the anticoagulant effects of BMS-177/JNJ-3093 in vitro. A clinical study is required to determine if these agents can reverse the anticoagulant effects of BMS-177/JNJ-3093 in patients.
Bunce:Johnson & Johnson: Current Employment, Current equity holder in publicly-traded company. Huang Devine:Johnson & Johnson: Current Employment, Current equity holder in publicly-traded company. Chintala:Johnson & Johnson: Current Employment, Current equity holder in publicly-traded company.
FEIBA: hemophilia A and B patients with inhibitors for: control and prevention of bleeding episodes; use around the time of surgery; routine prophylaxis to prevent or reduce the frequency of bleeding episodes NovoSeven: Treatment of bleeding and prevention of bleeding for surgeries and procedures in adults and children with hemophilia A or B with inhibitors, congenital Factor VII (FVII) deficiency, and Glanzmanns thrombasthenia with a decreased or absent response to platelet transfusions; treatment of bleeding and prevention of bleeding for surgeries and procedures in adults with acquired hemophilia Kcentra: urgent reversal of acquired coagulation factor deficiency induced by vitamin K antagonist therapy in adult patients with need for urgent surgery/invasive procedure or acute major bleeding Profilnine: prevention and control of bleeding in patients with Factor IX deficiency due to hemophilia B BeneFix: control and prevention of bleeding episodes or peri-operative management in adult and pediatric patients with hemophilia B Novoeight: for use in adults and children with hemophilia A for control and prevention of bleeding, perioperative management, and routine prophylaxis to prevent or reduce the frequency of bleeding episodes Cyklokapron: patients with hemophilia for short-term use to reduce or prevent hemorrhage and reduce the need for replacement therapy during and following tooth extraction)
Author notes
Asterisk with author names denotes non-ASH members.