Introduction: Children with sickle cell disease (SCD) frequently experience pain beginning in early childhood progressing throughout the lifespan. SCD-related pain during childhood is associated with decreased physical, psychological, social, and quality of life (QOL) outcomes. As a chronic, multi-organ condition, treatment for SCD may require complicated preventive health and acute treatment regimens necessitating development of self-management strategies for children and their families. mHealth may be an ideal mechanism for implementation of self-management strategies. A mHealth self-management intervention was developed with an end-user-based approach (Voice Crisis Alert V2) and guided by the Pediatric Self-Management model. Initial feasibility testing was conducted. This abstract describes preliminary impacts of the Voice Crisis Alert V2 intervention on symptoms and QOL.
Methods: The characteristics and names of application (app) components such as "Crisis Care" and "I'm in Pain" are outlined in Table 1. Targeted sample size was 60 dyads of children with SCD up to age 18 and the parent/caregiver. For assessment of impact, data sources included app use, child and parent/caregiver surveys at baseline, mid-intervention, end-of intervention and post-intervention. Survey instruments included the PROMIS SF for anxiety, fatigue, depressive symptoms, pain interference, pain intensity and sleep disturbance, and the PedsQL and Sickle Cell Disease Module (SCDM) for health-related quality of life (HRQOL). Data analysis was conducted using descriptive statistics, t-tests, Wilcoxon Signed Rank test, and Spearman's rho. Independent variables were number of times and length of time (minutes) using the app and individual app components. Dependent variables were pre-post differences in scores for total PedQL, school functioning, total SCDM, and subscales: pain and hurt, pain intensity, pain management, and treatments. Results are reported with 95% CIs.
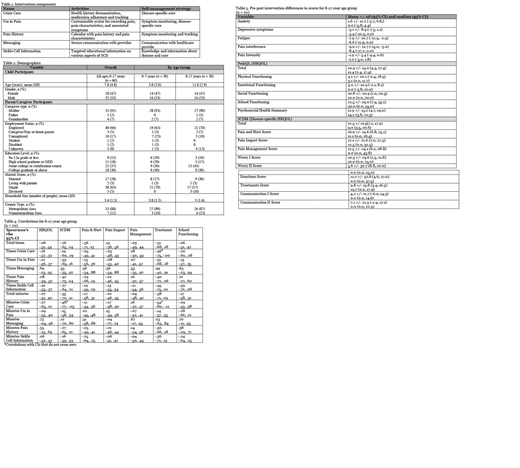
Results: The most substantial pre- to post-intervention differences in symptoms and QOL scores were noted in children ages 8 - 17. Pre-post-intervention differences in scores demonstrated improvement for all symptoms outcomes except anxiety, with clinically significant improvements in depressive symptoms, fatigue, and pain interference. Improvement in total scores and all subscale scores on the PedsQL and SCDM instruments were also noted from pre- to post-intervention. The majority of correlations had CIs that crossed zero, indicating either negative or positive associations may be observed in the population. Thus, overall, frequency and length of app use was not correlated with degree of improvement in outcomes. Negative correlations were observed between the number of times the Crisis Care component was used and treatment score (rs = -.46, 95% CI [-.75, -.00]), the number of minutes the Crisis Care component was used and the treatment score (rs = -.54, 95% CI [-.80, -.11]), and the number of minutes the Crisis Care component was used and the total SCDM score (rs = -.48, 95% CI [-.77, -.03]). These correlations suggest more frequent and longer use of the Crisis Care component was associated with less substantial improvement in SCDM total score and treatments score.
Conclusions: Greater improvement in scores for children ages 8 - 17 compared to children less than 8 years suggests the intervention may be particularly useful in older children with SCD. Changes in scores from pre- to post-intervention and their CIs suggest intervention use may lead to improvement in the following domains: fatigue, pain interference, HRQOL, physical functioning, social functioning, pain and hurt, pain impact, pain management, and treatments. While improvements in scores from pre- to post-intervention were noted in nearly all areas, few meaningful correlations were observed between app use and differences in outcome scores. Improvement in symptoms and QOL outcomes may be attributable to individual characteristics, such as disease severity, or the amount and type of app use required to develop self-management skills may be individualized. Sample sizes were small and future efficacy testing is warranted with a larger sample.
Kanter:AGIOS: Membership on an entity's Board of Directors or advisory committees; BEAM: Membership on an entity's Board of Directors or advisory committees; NHLBI Sickle Cell Advisory Board: Membership on an entity's Board of Directors or advisory committees; SCDAA Medical and Research Advisory Board: Membership on an entity's Board of Directors or advisory committees; Cowen: Honoraria; Wells Fargo: Honoraria; bluebird bio, inc: Consultancy, Honoraria; Novartis: Consultancy; Sanofi: Consultancy; Medscape: Honoraria; Guidepoint Global: Honoraria; GLG: Honoraria; Jeffries: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.