INTRODUCTION:
Dehydrated hereditary stomatocytosis (DHSt) is an autosomal dominant red blood cell membrane disorder characterized by hemolytic anemia and splenomegaly. DHSt has an estimated incidence of 1:50,000 births, and the degree of anemia varies within and between families. Although transfusion support during childhood is not uncommon, continued requirement into adulthood is rare. The most frequent cause of DHSt is a gain-of-function mutation of the PIEZO1 gene, leading to delayed channel inactivation that results in a monovalent cation leak and an increase in intracellular calcium (Ca2+). Many of these patients develop recurrent thromboses post splenectomy. Other DHSt patients have mutations in KCNN4, which encodes the Gardos channel, with mutations causing increased Ca2+ sensitivity and potassium efflux. To our knowledge, 42 patients from ten families have been described with four distinct KCNN4 mutations: Arg352His, Val282Met or Val282Glu, and a 28bp deletion encompassing the exon-intron 7 junction. We report herein the eighth family with the Arg352His locus mutation.
CASE REPORT:
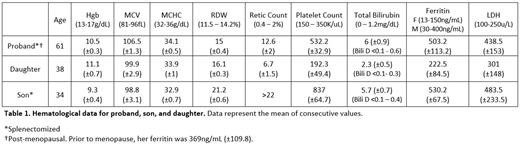
Five subjects from a single family were enrolled in this study (affected proband, unaffected husband, two affected children, and an unaffected grandchild). The proband has had hemolytic anemia since childhood. She had undergone splenectomy at age three and cholecystectomy at eight. Her anemia persisted, with a mean hemoglobin (Hgb) of 10.5g/dL and a reticulocyte count (retic ct) of 12.6%, with no need for transfusion support or iron chelation therapy (Table 1). Extensive testing revealed slightly decreased osmotic fragility and mildly elevated intracellular sodium concentration, of 19.4 mEq/L. Her daughter has mild splenomegaly and anemia, with a mean Hgb of 11.1g/dL and retic ct of 6.7%, whereas her son has more severe disease, with a mean Hgb of 9.3g/dL and a retic ct of >22%. He underwent splenectomy as a teenager for immune thrombocytopenia, and has required chelation therapy with deferasirox since age 31, when his ferritin rose to >1000ng/mL. Similar to results reported by others, splenectomy did not alter the severity of hemolysis in either the proband or her son, and neither developed thrombotic complications 57 and 7 years post splenectomy, respectively. The proband's daughter has a child with a normal Hgb, and is presumed unaffected. Stomatocytes were rarely seen on the peripheral blood smears of the proband and her children.
METHODS:
A clinically available 39 gene hemolytic anemia panel on the proband failed to identify the mutation underlying this disorder. We therefore performed whole exome sequencing on all five family members. We prioritized the analysis of 23 additional genes that are included in hemolytic anemia panels from two other reference laboratories and are involved in disorders of red blood cell membrane or cytoskeletal proteins of potential clinical relevance to the study population.
RESULTS:
A single missense mutation, Arg352His within KCNN4, was identified in all three affected individuals. This heterozygous mutation was present in the proband and her affected two children, and absent in her unaffected grandchild and husband.
CONCLUSIONS:
To our knowledge, this Pennsylvania family is only the eleventh described to have DHSt secondary to a KCNN4 mutation. This disorder is likely much more prevalent than reported, due to the rarity of stomatocytes on peripheral blood smears, the omission of the KCNN4 gene from hemolytic panels offered by some reference laboratories, and variable clinical presentation. KCNN4 mutations should be investigated if other causes are not identified in patients with lifelong hemolytic anemia suspected of having a red cell membrane protein or cytoskeletal disorder. Appropriate diagnosis may allow severely affected patients to be considered for treatment with the experimental Gardos channel inhibitor senicapoc. Additionally, mutational diagnosis is especially important when considering the adverse outcomes post splenectomy in PIEZO1 as compared to KCNN4 mutations.
Eyster:SPARK:Research Funding;Sanofi:Research Funding;Novo Nordisk:Research Funding;Baxalta/Shire:Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.