Introduction: Acute hepatic porphyria (AHP) is a family of rare genetic diseases due to enzyme defects in hepatic heme biosynthesis. Induction of 5-aminolevulinic acid synthase 1 (ALAS1), the rate-limiting step in heme biosynthesis, leads to accumulation of heme intermediates, 5-aminolevulinic acid (ALA) and porphobilinogen (PBG) that may result in neurovisceral attacks. ENVISION (NCT03338816) is an ongoing study, evaluating efficacy and safety of givosiran in symptomatic AHP patients in a 6-month double blind (DB) period and a 30-month open label extension (OLE) period. While the efficacy and safety profile of givosiran has previously been reported in the DB period, here its effect through Month 18 of the OLE period is reported.
Methods: ENVISION is an ongoing Phase 3 global, randomized, placebo-controlled study. Exploratory efficacy outcome measures in the OLE included composite porphyria attacks (i.e. those requiring hospitalization, urgent care, or IV-hemin at home), ALA/PBG levels and hemin use. In addition, quality of life assessments (Physical Component Summary Short Form-12 [PCS SF-12], EuroQoL Visual analog scale [EQ-VAS]), the Porphyria Patient Experience Questionnaire (PPEQ), and missed days of work were assessed. Analyses were descriptive and represent the timepoint after which all patients completed at least their Month 18 visit (01/10/2020).
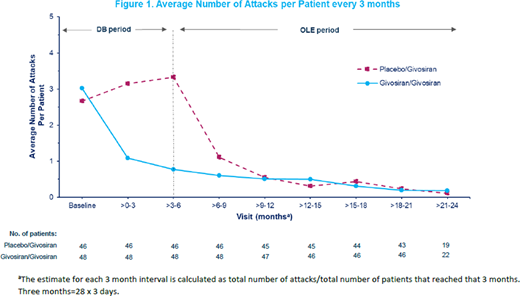
Results: As of January 10, 2020, 94 patients completed the DB period and 93 patients entered the OLE (placebo/givosiran=46; givosiran/givosiran=47). Mean exposure to givosiran at data cutoff was 12.97 [SD=3.6] months for placebo/givosiran and 18.86 [3.6] months for givosiran/givosiran, with maximum exposure of 25.1 months. Continued treatment in givosiran/givosiran patients led to a median annualized attack rate (AAR) of 0.58 (range: 0-16.2) through Month 18. Patients in the placebo/givosiran group had an AAR of 1.62 (range: 0-11.8) after receiving givosiran for ≥12 months during the OLE period, compared with 10.65 (range: 0-51.6) whilst receiving placebo during the 6-month DB period. The average number of attacks per patient over time following givosiran treatment continued to decline during the OLE period for both groups (Figure 1). Sustained ALA/PBG lowering during the OLE was accompanied by sustained reductions in hemin use, and more than half of the placebo/givosiran patients experienced 0 days of hemin use. Improvements in PCS SF-12 scores at Month 6 (mean change from baseline=+5.1 [SD=9.0]) were maintained at Month 18 (mean change from baseline=+7.0 [7.0]) for givosiran/givosiran patients, with similar improvements observed in placebo/givosiran patients at Month 18 (mean change from baseline=+9.9 [8.2]). Continued givosiran treatment in givosiran/givosiran patients led to further improvements in EQ VAS compared with DB period (mean change from baseline 5.2 at completion of the DB period [SD=22.2] and 13.7 [22.5] at Month 18 during the OLE period), with placebo/givosiran patients also showing improvements at Month 18 (mean change from baseline 8.3 [SD=18.5]). Placebo/givosiran patients reported improvements in PPEQ scores (traveling, social activities, planning future events, household chores, exercise, and treatment satisfaction) since initiating givosiran, comparable to the improvement observed in the givosiran group during the DB period. Additionally there was a decrease in the number of work days missed due to porphyria in the past 4 weeks at Month 6 (mean=6.7 days [SD=7.8], n=20/46) compared with Month 18 (2.5 [5.1], n=23/46), for patients in the placebo/givosiran group who were able to work. The most common related adverse events (AEs) occurring during givosiran treatment were injection site reactions, nausea and fatigue. Hepatic and renal events were both reported in 17% of patients each during givosiran treatment. No new safety concerns occurred in the OLE compared with DB period.
Conclusion: In the ongoing OLE period of the ENVISION study, patients receiving long-term treatment with givosiran demonstrated a durable response in clinical efficacy, across a wide range of clinical parameters. Following the initial 6 months of givosiran treatment during the OLE, placebo/givosiran patients had a similar clinical response to that observed in givosiran/givosiran patients in the OLE period through Month 18. The safety profile of givosiran remained acceptable and consistent with that previously observed.
Kuter:Bristol-Myers Squibb: Consultancy, Honoraria, Other: Travel Expenses, Research Funding; Dova: Consultancy, Honoraria; Momenta: Consultancy, Honoraria; Argenx: Consultancy, Honoraria, Other: Travel Expenses, Research Funding; Agios: Consultancy, Honoraria, Other: Travel Expenses, Research Funding; Daiichi Sankyo: Consultancy, Honoraria; Actelion (Syntimmune): Consultancy, Honoraria, Other: Travel Expenses, Research Funding; Protalex: Consultancy, Honoraria, Research Funding; Shionogi: Consultancy; UCB: Consultancy, Honoraria; Platelet Disorder Support Association: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Caremark: Consultancy, Honoraria; CRICO: Consultancy, Honoraria; Immunovant: Other: Travel Expenses, Research Funding; Kezar Life Sciences, Inc: Other, Research Funding; Principia Biopharma: Consultancy, Honoraria, Other, Research Funding; Protalex: Consultancy, Honoraria, Other, Research Funding; Sanofi (Genzyme): Consultancy, Honoraria; Genzyme: Consultancy, Honoraria; Immunovant: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Kyowa-Kirin: Consultancy, Honoraria; Merck Sharp Dohme: Consultancy, Honoraria; Protalix Biotherapeutics: Consultancy; Principia: Consultancy, Research Funding; Shire: Consultancy, Honoraria; Shionogi: Consultancy, Honoraria; Up-To-Date: Consultancy, Honoraria, Patents & Royalties; Zafgen: Consultancy, Honoraria; Takeda (Bioverativ): Consultancy, Honoraria, Other, Research Funding; Rigel: Consultancy, Honoraria, Other, Research Funding; Alnylam: Consultancy, Honoraria, Other: Travel Expenses, Research Funding; Amgen: Consultancy, Honoraria, Other: Travel Expenses, Research Funding. Rees:AstraZeneca: Other: Data monitoring committee membership; Novartis: Consultancy, Honoraria; TauRx: Other: Data And Safety Monitoring; Alnylam Pharmaceuticals: Consultancy, Honoraria; Emmanus Medical: Consultancy, Honoraria. Ventura:Alnylam Pharmaceuticals: Consultancy, Honoraria; Recordati Rare Diseases: Consultancy, Honoraria. Balwani:Recordati Rare Diseases: Consultancy, Honoraria, Other: Disease information video recording; Alnylam Pharmaceuticals: Consultancy, Honoraria, Research Funding. Gouya:Alnylam Pharmaceuticals: Consultancy, Honoraria, Other: Scientific meeting. Simon:Alnylam Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Liu:Alnylam Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Ko:Alnylam Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Rhyee:Alnylam Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Silver:Alnylam Pharmaceuticals: Other: Travel reimbursement, Research Funding; Blue Care Network: Consultancy; Oncology Business Review: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.