Background:Internal tandem duplications (ITDs) within the FMS-like tyrosine kinase 3 (FLT3) gene (FLT3-ITD) is a common driver mutation and confers a poor prognosis in patients (pts) with acute myeloid leukemia (AML) due to proliferative presentation, short remission durations and early relapse. The addition of midostaurin to induction therapy has become standard therapy in newly diagnosedFLT3-mutated (FLT3-mu) AML. Next-generation FLT3 inhibitors (FLT3i) as gilteritinib and quizartinib are more specific, more potent, and may have fewer toxicities associated with off-target therapies but are only available in clinical trials or for salvage therapy. Real-world experience in smaller academic institutions is scanty.
Methods:We reviewedFLT3-ITD mu AML pts at our institution from October 2016-December 2019. FLT3i were given as a single agent or in combination with cytotoxic chemotherapy (CCT) or low intensity therapy (LIT) (hypomethylating agents). AllFLT3-ITD DNA was subjected to PCR amplification using primers targeting the juxtamembrane domain of FLT3, and products were analyzed by capillary electrophoresis. We analyzed the characteristics of these pts, responses to therapy, and outcomes.
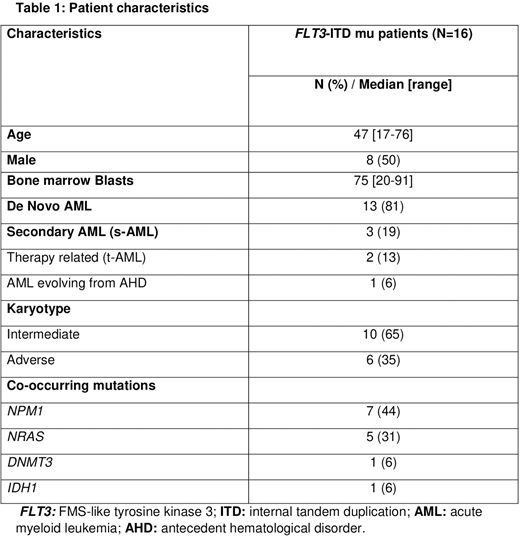
Results:Sixteen pts with newly diagnosedFLT3-ITD mu AML were identified. The median age was 47 years [range, 17-76 years] and the median follow-up was 9.3 months [range, 1.2-29.5 months]. The median time from AML diagnoses to FLT3 detection was 4 days [range, 3-5 days]. Patient characteristics are shown in Table 1. The most common co-occurring mutations wereNPM1(44%) andNRAS(31%); 2 pts (12%) had both mutations. Five pts (31%) received CCT and 11 pts (69%) received CCT+FLT3i. The most frequently used FLT3i was midostaurin 25 mg twice daily on days 8 through 21 (14 days) (10 pts, 63% of all pts); 1 pt received sorafenib (6% of all pts). The complete remission rate (CR) and the overall response rate (ORR) (CR+CR with incomplete hematologic recovery [CRi]) were 31% and 88%, respectively. The two pts who did not achieve a remission were older than 65 years. Among the pts who received CCT+FLT3i, CR and OOR were 50% and 100%, respectively. The median number of cycles to remission was 1 [range, 1-2 cycles], and the median number of consolidation cycles was 0 [range, 0-3 cycles]. Overall, the median duration of response in frontlineFLT3-ITD pts who did not receive allogeneic hematopoietic stem cell transplantation (Allo-HSCT) was 5 months [range, 1-9 months] (all of them received CCT+FLT3i). There was no statistically significant difference in response in pts with NPM1 mutation compared with pts with NRAS mutation (P=0.7), or with no additional mutations. Seven pts (44%) received an Allo-HSCT (6 after CCT+FT3i, and 1 after CCT alone): 2 (28%) from matched unrelated donor, 1 (14%) from a haploidentical transplant, 1 (14%) from dual cord transplant, and the remaining (44%) from matched related donors. Two patients relapsed after Allo-HSCT (28%). Both of these patients had received FLT3i before Allo-HSCT; one of the two relapsed pts received ivosidenib and azacitidine (IDH1 mutation) after Allo-HSCT, achieved remission then received the 2nd Allo-HSCT with subsequent remission. Two pts (29%) received maintenance therapy after Allo-HSCT, one with midostaurin, and the other with azacitidine + gilteritinib; both pts are still on remission (24 and 6 months after CR, respectively). All 7FLT3-ITD pts who did not receive Allo-HSCT relapsed. Gilteritinib was used as a single agent (n=3) or in combination with LIT (n=1) in these pts after relapse. CR rate was 25% and CRI 50%; one pt relapsed (33%). All pts who responded are still receiving gilteritinib.
Conclusion:Combination of FLT3i based therapy with CCT is effective therapy inFLT3-ITD frontline pts in real-world setting. Duration of response is short in the absence of Allo-HSCT.
Cortes:Arog:Research Funding;Pfizer:Consultancy, Research Funding;Amphivena Therapeutics:Research Funding;Daiichi Sankyo:Consultancy, Research Funding;Astellas:Research Funding;Telios:Research Funding;Novartis:Consultancy, Research Funding;BioPath Holdings:Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding;Merus:Research Funding;Bristol-Myers Squibb:Research Funding;Takeda:Consultancy, Research Funding;Sun Pharma:Research Funding;BiolineRx:Consultancy, Research Funding;Immunogen:Research Funding;Jazz Pharmaceuticals:Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.