With improved understanding and treatment of sickle cell disease (SCD), patients are more likely to live into the sixth decade. Survival into older adulthood may be associated with a concomitant risk of malignancy in this population. Past studies have yielded conflicting data regarding the incidence of cancer in SCD. Some studies show an increased risk of hematologic malignancies or solid tumors, while others demonstrate a decreased propensity for cancers in patients with SCD. Delays in treatment and dose reductions can adversely affect response and survival outcomes, but little is known about the ability of SCD patients to tolerate treatment for various malignancies.
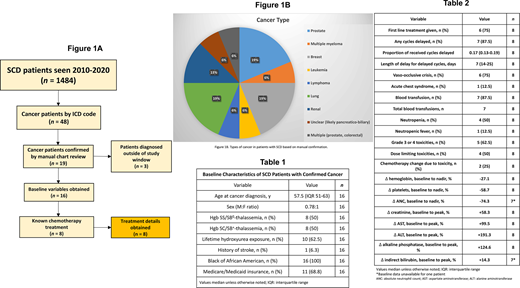
We studied the incidence of cancer and tolerance of chemotherapy over a 10-year-period at the University of Illinois at Chicago in a retrospective chart review. Our study included 1484 SCD patients of all ages seen at our institution from September 30, 2010 to April 29, 2020. The patient records were queried for common malignancies using ICD-9 and ICD-10 codes, including colorectal, lung, breast, prostate, renal, and cervical cancer as well as lymphoma, leukemia, and multiple myeloma. These cases were verified by manual chart review (Figure 1A). The incidence of cancer was compared to an African American (AA) control cohort of 22,260 patients matched for age, sex, and follow-up time. For patients who received chemotherapy, details on regimen and tolerability were obtained. Baseline variables were defined as the median of all available values three years prior to treatment. Dose limiting toxicities (DLT) were defined as any reason for which an expected dose could not be administered.
Of the 1484 SCD patients, 48 (3.2%) had a cancer diagnosis according to ICD codes. Among the AA control population, 1282/22260 (5.8%) had cancer according to ICD codes. The incidence of common malignancies was significantly decreased in the SCD population (OR 0.55, p<0.001, 95% CI 0.40 to 0.73). Of the 48 SCD cancer cases, we were able to confirm 19 (39.6%) by manual chart review and 16 of these patients were diagnosed during the study period. Characteristics of the 16 SCD cancer patients are summarized in Table 1 and distribution of cancer types is shown in Figure 1B. Eight (50%) of these patients were known to receive chemotherapy, and treatment details are shown in Table 2. First-line treatment was not given in 2 (25%) cases due to comorbidities such as SCD, poor functional status, toxicity, age, and renal insufficiency. Grade 3 or 4 toxicities took place in 5 (62.5%) patients, including anemia, neutropenia, leukopenia, thrombocytopenia, dehydration, nausea and or vomiting, and hemorrhagic cystitis. Six (75%) patients had a vaso-occlusive crisis (VOC) and 1 (12.5%) had an acute chest syndrome episode during therapy. DLT in 4 (50%) patients included nephrotoxicity, infection, pain, and hepatotoxicity. Four (50%) patients did not receive all scheduled cycles for reasons including concern for cerebrovascular accident (CVA), fatigue, anorexia, dehydration, VOC, infection, and progression of disease. Two (25%) patients required change in chemotherapy regimen due to toxicity.
Our study raises several questions regarding both the incidence and treatment of cancer in SCD. There was a significantly lower incidence of cancer among SCD patients compared to AA controls. Mechanisms by which SCD patients may be protected from cancer are unknown, but should be explored in future studies. Patients who received chemotherapy in our study experienced significant toxicities, delays, and dose reductions. Such issues were often SCD-related or due to other typical hematologic toxicities. Although the goal for treating SCD cancer patients may be with therapies that result in outcomes similar to the general population, our data suggests that this is difficult with routine protocols. Increased monitoring and a multimodal approach to symptom control may increase treatment tolerability and ensure adequate dose delivery. Evidence of poor chemotherapy tolerance in SCD patients highlights the need for further and larger studies to determine treatment strategies for this population.
Saraf:Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Boards, Speakers Bureau; Novartis, Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees; Pfizer, Global Blood Therapeutics, Novartis: Research Funding. Gordeuk:Novartis: Consultancy; CSL Behring: Consultancy, Research Funding; Global Blood Therapeutics: Consultancy, Research Funding; Imara: Research Funding; Ironwood: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.