Introduction
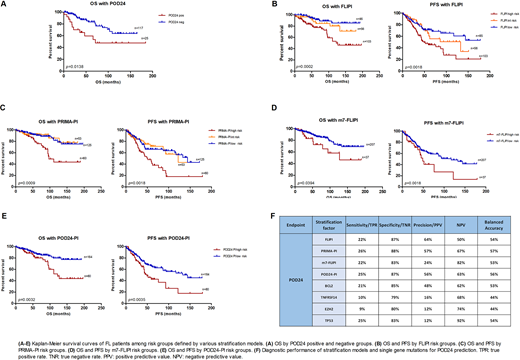
Progression of disease within 2 years after starting rituximab-chemotherapy (POD24) has been identified as a convincing adverse prognostic factor for follicular lymphoma (FL), and can serve as a clinical endpoint to identify patients at high risk of early lymphoma-related mortality. However, POD24 is not accessible at diagnosis. Several stratification models adopting baseline variables have been developed for predicting outcomes in FL patients. The Follicular Lymphoma International Prognostic Index (FLIPI) and PRIMA Prognostic-Index (PRIMA-PI) include clinical characteristics, while m7- Follicular Lymphoma International Prognostic Index (m7-FLIPI) and POD24 Prognostic Index (POD24-PI) integrate gene mutations with clinical features. Moreover, the mutation status of specific genes (including TP53, EZH2, TNFRSF14, and BCL2) has been reported to be relevant to early progression. It has been reported that m7-FLIPI and POD24-PI are predictive for POD24. Here we compare the diagnostic accuracy for POD24 of various stratification models and gene mutations in an institutional cohort of FL.
Methods
Consecutive patients diagnosed as FL grades 1-3a were enrolled from the Oncology Institute of Southern Switzerland (n=75), the University of Eastern Piedmont (n=118), and the Hematology of the AUSL IRCCS of Reggio Emilia (n=59). DNA was extracted from FFPE tissue specimen obtained at diagnosis. We performed CAPP-seq to detect the mutation status of the genes included in m7-FLIPI and POD24 PI (EZH2,ARID1A, MEF2B, EP300, FOXO1, CREBBP, and CARD11), as well as BCL2, TNFRSF14 and TP53. POD24 was the primary endpoint of this research. Sensitivity, specificity, predictive values and balanced accuracy of every stratification model were calculated to estimate the prognostic efficacy.
Results
With FLIPI score, 34% of patients were classified as low risk, 22% as intermediate risk, and 41% as high risk. With PRIMA-PI, the fractions for low risk, intermediate risk and high risk group were 24%, 21% and 49%, respectively. With m7-FLIPI, 82% of patients were low risk, and 15% high risk. For POD24-PI, 65% of patients were low risk, and 32% high risk. The mutation frequencies of TP53, TNFRSF14, EZH2 and BCL2 were 8%, 28%, 27% and 38%, respectively. We validated the prognostic utility of FLIPI, PRIMA-PI, m7-FLIPI and POD24-PI for progression free survival (PFS) and OS. However, no statistically significant relevance with PFS or OS has been found for TP53, TNFRSF14, EZH2 or BCL2 mutations. POD24 was calculated in the 142 patients who received systemic treatment (immunotherapy or chemoimmunotherapy) within 6 months from diagnosis: 18% of them were POD24-positive. POD24 positivity associated with shorter overall survival (OS) (p=0.0138). After adjusting for multiple comparisons, no high-risk groups identified by TNFRSF14, EZH2 or BCL2 gene mutations, FLIPI, PRIMA-PI, m7-FLIPI or POD24-PI were associated with POD24. In terms of diagnostic performance for POD24, PRIMA-PI showed the highest accuracy (57%), FLIPI had the highest positive predictive value (64%), and m7-FLIPI had the highest negative predictive value (82%).
Conclusion
The mutation status of specific single genes were not related to POD24, PFS nor OS, indicating that the mutation of single gene may not be sufficient to identify high-risk FL patients. Though retaining their prognostic value, the integration of mutations onto the clinical biomarker-based prognostic scores has a limited discrimination capacity for POD24. Our results prompt the investigation of: i) POD discrimination capacity of prognostic models based on molecular phenotypes (i.e. gene expression) reflecting the tumor-microenvironment milieu; ii) new models based on a combination of biomarkers capturing the most informative clinical, genetic and phenotypic features.
Moccia:Takeda: Consultancy, Other: Advisory Boards: Roche, Janssen, Takeda; Roche: Consultancy, Other: Advisory Boards: Roche, Janssen, Takeda; Janssen: Consultancy, Other: Advisory Boards: Roche, Janssen, Takeda. Stathis:ADC Therapeutcis: Other, Research Funding; Abbvie: Other: Travel Grant; MEI Pharma: Other, Research Funding; Novartis: Other, Research Funding; Roche: Other, Research Funding; Pfizer: Other, Research Funding; PharmaMar: Other: Travel Grant; Cellestia: Research Funding; Loxo: Honoraria, Other, Research Funding; Member of the steering committee of the trial of this abstract: Other; Bayer: Other, Research Funding; Merck: Other, Research Funding. Gerber:Alnylam: Other: funding for accredited continuing medical education; Axonlab: Other: funding for accredited continuing medical education program ; Bayer: Other: funding for accredited continuing medical education program ; Bristol Myers Squibb: Other: funding for accredited continuing medical education program ; Daiichi-Sankyo: Other: funding for accredited continuing medical education program ; Janssen: Other: funding for accredited continuing medical education program ; Mitsubishi Tanabe Pharma: Other: funding for accredited continuing medical education program ; NovoNordisk: Other: funding for accredited continuing medical education program ; Octapharma: Other; Takeda: Other; Sanofi: Other; SOBI: Other; Thermo Fishe: Other; Axonlab: Other; Pfizer: Other: personal fees ; Sanofi: Other: funding for accredited continuing medical education. Gaidano:Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astrazeneca: Membership on an entity's Board of Directors or advisory committees; Sunesys: Membership on an entity's Board of Directors or advisory committees. Rossi:Gilead: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding. Zucca:Roche: Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants, Research Funding; Celltrion Healthcare: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Abbvie: Other: Travel Grants; Beigene: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.