Introduction: Current evidence suggests that the COVID-19 related mortality and morbidity is higher in cancer patients due to increased visits to healthcare facilities (e.g., doctor's visits, phlebotomy, imaging, social work, financial consultations, therapy administration, etc) and inherent or therapy-induced immunosuppression. Patients with hematologic malignancies harboring SARS-CoV-2 have the highest mortality compared with other cancer patients. Although COVID-19 is knowingly transmitted person to person via respiratory droplets, there is potential for infection from contact with surfaces (e.g. plastic, metal, cardboard, etc) polluted with fomites that have been shown to have viable virus up to 72 hours in laboratory settings (Van Doremalen et al. NEJM 2020). The risk of nosocomial infections is hypothesized to be due to environmental contact and contamination, but there are no real-world analyses that exist that demonstrate the impact of recently established infection control policies (e.g., strict use of personal protective equipment (PPE), social distancing, hand hygiene, disinfectant protocols, etc) in hematology/oncology patient settings. This study evaluated the frequency of SARS-CoV-2 on a multitude of environmental surfaces in outpatient and inpatient settings in a large tertiary COVID-19 referral cancer center in New Jersey.
Methods: IRB approval was obtained to conduct the study in a COVID-19 referral center in 2 large, freestanding outpatient clinics (i.e., malignant hematology and medical oncology) and co-localized infusion suites as well as two physically separated inpatient units (i.e., a leukemia/lymphoma/CART unit and an active COVID-19 floor, the latter housing cancer patients actively infected with SARS-CoV-2 and persons under investigation (PUI) for infection). Surface testing for viral RNA in the outpatient infusions suites included spaces where several patients with recent SARS-CoV-2 infection were receiving cancer treatment in the outpatient setting. High-impact areas were selected based on frequency of use, patient and healthcare provider contact, and risk of contamination from COVID-19 positive subjects (COVID-19+) and PUIs due to virus transmissibility. Surfaces were sampled on Mondays, Wednesdays and Fridays from 6/17/20 through 6/29/20 prior to scheduled cleaning and disinfection services, but after patient or staff use, following WHO protocols for COVID-19 surface sampling. Specimens were analyzed with real-time reverse transcriptase PCR analysis.
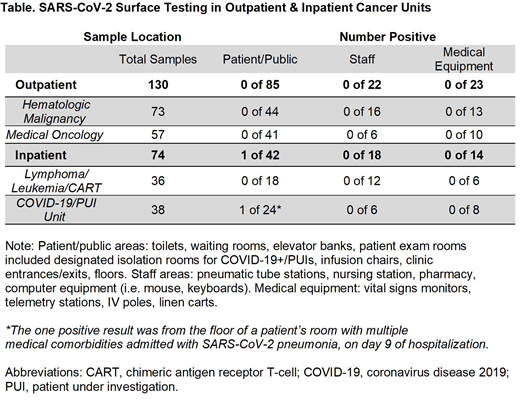
Results: Overall, 204 total environmental samples were collected over the study period. Testing sites were categorized as 1) Public areas (waiting rooms, infusion areas, bathrooms, floors, elevator banks, doors, and exam rooms included designated isolation rooms for known COVID-19+/PUIs); 2) Staff areas (computer equipment, pneumatic tubing stations, pharmacy bench, and medication rooms); and 3) Medical equipment (IV poles, chemotherapy bags, vitals monitor, telemetry stations, and linen carts).
Among the 130 surfaces examined from the two outpatient hematology-oncology clinics and 36 tests from the inpatient leukemia/lymphoma/CART cell unit, all 166 surfaces were negative for SARS-CoV-2 viral RNA (see Table). Among the inpatient COVID-19+ and PUI units, one of 38 samples were positive (2.6%). The one positive sample was taken from the floor of an elderly patient with multiple medical comorbidities being treated with remdesevir, dexamethasone, and apixaban for SARS-CoV-2 pneumonia. Altogether, the positive test rate for SARS-CoV-2 RNA across all surfaces in the combined outpatient and inpatient hematology/oncology units was 0.5% (1/204).
Discussion: This systematic investigation of 'real world' environmental surfaces performed in outpatient and inpatient hematology-oncology units revealed overall negligible detection of active SARS-CoV-2 RNA. It highlights the efficacy of current, detailed infection control policies (e.g., screening, nursing-directed triage for PUIs/COVID-19+ patients, visitor restriction, telehealth, social distancing, and disinfection protocols) and should also mitigate concerns of healthcare providers and patients, particularly those in the healthcare setting with blood disorders. Additionally, the one positive sample from a COVID-19 unit emphasizes the need for physical separation of patients with SARS-CoV-2.
Evens:Research To Practice: Honoraria, Speakers Bureau; Merck: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria, Research Funding; Epizyme: Consultancy, Honoraria, Research Funding; Mylteni: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.