Introduction: Patients with relapsed/refractory acute myeloid leukemia (R/R AML) have limited therapeutic options and effective management remains clinical challenge. For medically fit patients, salvage therapy with an intensive chemotherapy regimen is the standard of care with remission rates of about 40-50%. Long-term survival is achievable only with disease control followed by allogeneic hematopoietic stem cell transplantation (HCT). A majority of patients with R/R AML who achieve a morphologic clinical remission with salvage chemotherapy frequently harbor detectable clonal events that act as a reservoir for disease relapse. Relapsed/refractory isocitrate dehydrogenase 2 (IDH2) mutant AML patients who undergo cytarabine based salvage chemotherapy will largely retain detectable IDH2 mutations and enasidenib maintenance therapy offers an attractive and tolerable approach to maintenance for such patients. Enasidenib (AG221) is a first-in-class, selective, potent inhibitor of the neomorphic activity of mutant IDH2 enzyme. It is FDA approved as monotherapy for R/R AML with a time to response of approximately 2 months, but there is limited data on the use of this agent following salvage chemotherapy. In the upfront setting, the combination of enasidenib with induction chemotherapy has demonstrated high response rates, providing rationale for this approach (and tolerability with the combination) in the R/R population.
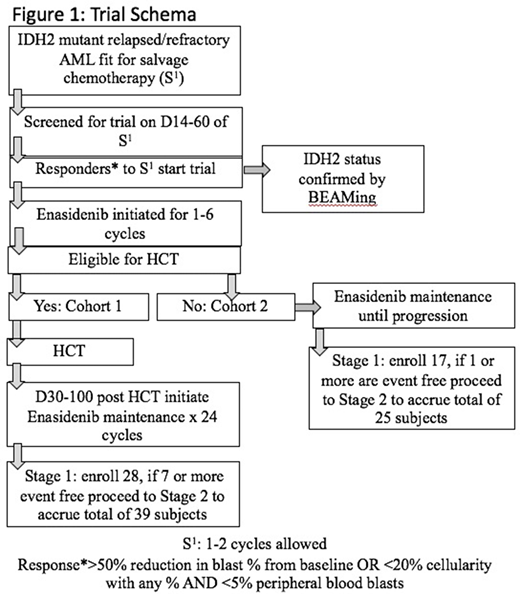
Methods: To understand the impact of tailored consolidation following salvage chemotherapy in fit R/R AML we designed a multi-center, phase II trial with 2 cohorts. Cohort assignment is based upon the investigators assignment of patients eligibility (cohort 1.) for allogeneic HCT or (cohort 2) no allogeneic HCT. The main inclusion criteria are, (a.) age over 18 years (b.) received salvage cytarabine based chemotherapy or venetoclax combined with hypomethylating agents (HMA) or 2 or more cycles of HMA for confirmed R/R AML (c.) achieved at least >50% reduction in blast percentage in a nadir marrow from salvage chemotherapy and (d.) adequate organ function and lack of acute life threatening illness. Patients will have received the investigator's choice of salvage therapy and subsequently undergo bone marrow assessment with protocol sampling. Patients confirmed to have obtained a remission will then begin enasidenib 100mg once a day starting between 14 and 60 days after starting salvage therapy. Patients in cohort 1 will receive 1-4 cycles of enasidenib and proceed with HCT; enasidenib can be re-initiated between D30-100 following HCT for a planned total of 24 cycles. Cohort 2 will continue enasidenib maintenance until progression/death or relapse (Schema: Fig 1).
Study Endpoints: The primary endpoint of the study to determine the effect of enasidenib maintenance therapy following salvage induction therapy for IDH2 R/R AML, we will evaluate EFS at 12 months separately in the two cohorts of the study. Secondary endpoints include (1) Rate of successful HCT of the subjects enrolled on the study (2) Median duration of maintenance therapy in both the study arms & (3) Overall survival at 12 and 24 months in each cohort. The efficacy of the treatment will be assessed using Simon (1989) optimal two-stage, single arm, unblinded Phase II trials allowing for early termination for futility. This design has a 90% power to detect a 20% improvement rate compared to historical data, while controlling to 10% probability of erroneously findings. A total of 39 (C1) + 25 (C2) = 64 evaluable patients will be enrolled. Interim safety analysis will be done after enrollment of the first 28 patient in C1 and 17 patients in C2.
Conclusion: This study is currently is open and enrolling patients at multiple collaborative institutions to answer the key question of efficacy of enasidenib maintenance following salvage chemotherapy. Correlative studies are being performed to understand minimal residual disease in IDH2 mutant AML and its clonal architecture.
Carraway:BMS: Consultancy, Other: Research support, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; ASTEX: Other: Independent Advisory Committe (IRC); Abbvie: Other: Independent Advisory Committe (IRC); Takeda: Other: Independent Advisory Committe (IRC); Stemline: Consultancy, Speakers Bureau; Jazz: Consultancy, Speakers Bureau. Griffiths:Novartis: Honoraria, Research Funding; Boston Biomedical: Honoraria; Genentech Inc: Research Funding; Astex Pharmceuticals: Research Funding; Alexion Pharmaceuticals: Honoraria, Research Funding; Celgene/BMS: Honoraria, Research Funding; AbbVie Inc: Honoraria; Persimmune: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.