Introduction: Coagulopathic bleeding is a frequent complication of cardiac surgery and can lead to excessive blood loss, blood product transfusion, and bleeding-related complications. Hemostatic management in bleeding surgical patients is evolving towards targeted therapy with purified products undergoing pathogen reduction technology. Unlike frozen plasma (FP), purified prothrombin complex concentrates (PCCs) do not require ABO blood type matching or thawing, are pathogen-reduced, and are associated with a lower risk of transfusion-associated circulatory overload and transfusion-related acute lung injury. The FARES study will compare the efficacy and safety of PCC (Octaplex; Octapharma) versus FP in bleeding cardiac surgical patients with confirmed or suspected coagulopathy. Preliminary non-randomized studies suggest PCC may be superior to plasma for management of bleeding after cardiac surgery.
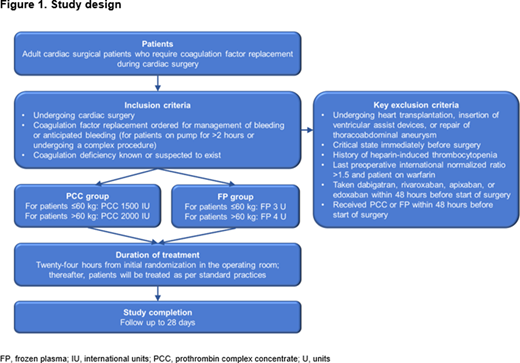
Methods: The randomized, active-control, single-blinded, pragmatic, investigator-initiated, Phase 2 FARES pilot study will enrolled 120 bleeding adult cardiac surgery patients requiring coagulation factor replacement from two hospitals to achieve 100 treated patients. The study outline is shown in Figure 1. Patients were randomized to PCC or FP, with two weight-based doses, for management of bleeding where coagulation factor deficiency is known or suspected (patients over 50 kg were transfused either 2000 IU of PCC or 4 units of plasma; patients under 50 kg were transfused 1500 IU of PCC or 3 units of plasma). Required second doses will be given according to randomization group allocation, but all patients will receive FP for their third and any subsequent doses. The primary endpoint is hemostatic effectiveness and transfusion requirements. Adverse events will be recorded from the beginning of surgery.
Results: The study commenced in September of 2019 and completed enrollment of the last patient in June of 2020. Results were monitored and overseen by the Independent Data and Safety Monitoring Committee. The database will be locked in August 2020 after 28 day follow-up is complete for all patients. Statistical analysis plan is complete. The results will inform a definitive, pragmatic, multi-center trial to determine if PCCs can replace plasma for the management of coagulopathy in the bleeding cardiac surgery patient.
Conclusions: This study will determine the feasibility, and inform the design and primary outcome parameter, of a definitive Phase 3 trial comparing the efficacy and safety of PCC versus FP in bleeding cardiac surgical patients requiring coagulation factor replacement. The pilot study results will also be used in sample size calculations and to aid detection of any safety issues.
Karkouti:Octapharma: Research Funding; Canadian blood services: Research Funding. Callum:Canadian Blood Services: Research Funding; Octapharma: Research Funding.
Coagulation factor replacement with Prothrmobin Complex Concentrates (off-label in the USA and on label in Canada/Europe)
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract