Background:
Cytomegalovirus (CMV) is a major cause of morbidity in allogeneic hematopoietic stem cell transplant (HSCT) patients. Marty et al. 2017 showed that letermovir is effective in preventing CMV reactivation in high-risk HSCT patients, though only 16% were haplo-identical. Recently, Karam et al. 2019 showed decreased rates of CMV reactivation in haplo-identical HSCT using letermovir in unselected high-risk patients. The effects of letermovir on other transplant-related outcomes including overall survival (OS), relapse free survival (RFS), and Graft-versus-host-disease (GVHD)-free-/relapse-free survival (GRFS), however, are not as well-known. With the increased use of post-transplant cyclophosphamide (PTCy) as GVHD prophylaxis in all transplant types, letermovir use may need to be broadened as those that are T-cell depleted are also at increased risk of CMV reactivation.
Methods:
We retrospectively analyzed adult patients at USC Norris Cancer Hospital (age ≥ 18) who received allo-HSCT from 2018 to 2020. Recipients who were CMV positive, received T-cell depleting therapies such as PTCy for GVHD prophylaxis and/or ATG in the conditioning regimen, and those who fulfilled the criteria in Marty et al. 2017 were categorized as high-risk. Patients were considered to have CMV reactivation if they had clinically significant serum CMV viremia or organ involvement by day+100. Letermovir was initiated on day+21 for high-risk patients. The primary end-point assessed was day+100 CMV reactivation. Secondary end-points included 1-year OS, 1-year RFS, 1-year transplant-related mortality (TRM), and 1-year GRFS, defined as grade 3-4 acute GVHD, systemic therapy-requiring chronic GVHD, relapse, or death in the 1-year post-HSCT period.
Results:
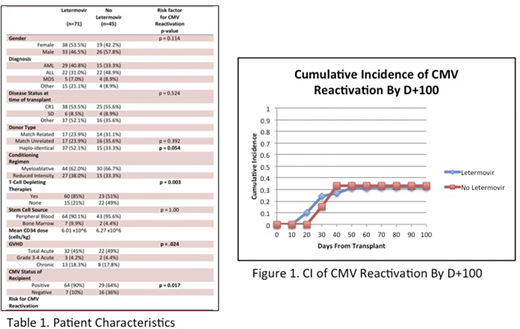
A total of 116 adult HSCT recipients were reviewed. 51% were male and 49% were female. The donor sources comprised of 27% match related, 28% match-unrelated, and 49% haplo-identical. Most common diseases included AML (38%), ALL (38%) and MDS (8%). 64% received myeloablative conditioning regimens while 36% received reduced intensity regimens. Furthermore, 92% of patients received peripheral blood with 8% receiving bone marrow. 70% of patients at time of transplant were in CR1 or had stable disease. 61% of patients received letermovir prophylaxis (n=71), all high-risk, and 39% did not (n=45). 13 high-risk patients did not receive letermovir due to insurance limitations and were included in the non-letermovir group. 85% (n =60) received T-cell depleting therapies in the letermovir group compared to 51% (n=23). 90% (n=64) were CMV positive in the letermovir group and 64% (n= 29) were positive in the non-letermovir group. Both groups were similar in regard to the incidence of GVHD. The only factors significantly associated with CMV reactivation were haplo-HSCT, CMV recipient positivity, use of PTCy as GVHD prophylaxis and/or ATG in conditioning regimen, risk status, and GVHD (p = 0.054, p = .017, p = 0.003, p = 0.050, and p = 0.024 respectively). In a subset analysis of all high-risk patients, CMV reactivation was 32% in the letermovir group and 69% in the non-letermovir group (p = 0.03). All other factors were not significantly associated with outcomes. CMV reactivation in the letermovir group was (32%) compared to the non-letermovir group (29%). 1-year OS, 1-year TRM, 1-year RFS, and 1-year GRFS were 85%, 13.2%, 87%, and 60% respectively in the letermovir group compared with 88%, 10%, 90%, and 56% in the non-letermovir group, though not statistically significant.
Discussion:
Our study confirms improved CMV reactivation outcomes with use of letermovir in high-risk patients. Furthermore, all end-points were similar between both groups, despite increased usage of myeloablative conditioning overall and more high-risk patients in the letermovir group, indicating improved 1-year OS, TRM, and GRFS compared with historical outcomes. Although the primary and secondary end-points were not statistically significant, it may be clinically meaningful, as our center has demonstrated similar outcomes amongst those who were high-risk and received letermovir and those who were low-risk and did not. This suggests broadened use of letermovir may be needed in the prevention of CMV reactivation and the improvement in overall outcomes. Further studies using letermovir in low risk patients may be necessary to investigate improved outcomes in the allo-HSCT setting.
Yaghmour:Jazz: Consultancy, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Speakers Bureau; Alexion: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.