BACKGROUND
Waldenström Macroglobulinaemia (WM) is an indolent, IgM-producing lymphoplasmacytic lymphoma (LPL) that infiltrates bone marrow (BM) and other tissues. Only symptomatic patients (with IgM-related complications, cytopenias, constitutional symptoms, bulky extramedullary disease) require treatment. Symptomatic BM lesions are not a recognised manifestation of WM and, if present, may raise the possibility of high-grade transformation.
AIMS
To characterise the clinical, radiological and histological features of focal bone marrow lesions (FBML) identified in WM patients with unrelenting pain in bones and/or joints, and outline implications for treatment.
METHODS
This study retrospectively reviewed investigations performed at the time of FBMLpresentation, including MRI of the site of pain, total body FDG-PET/CT, review of histology from BM trephine biopsy of the posterior superior iliac spine (PSIS) and CT-guided core biopsy of the identified FBML, and routine blood panels.
RESULTS
The 6 patients identified (Table 1) presented with localised skeletal pain at different stages of their WM disease course: 1 at initial WM diagnosis, 1 after 10 years of observation, 3 in remission following R-chemotherapy, and 1 during active treatment with R-chemotherapy. Median age at diagnosis of WM was 57 years (41-64 years). Median time from diagnosis to presentation with FBML was 28 months (0-120 months).
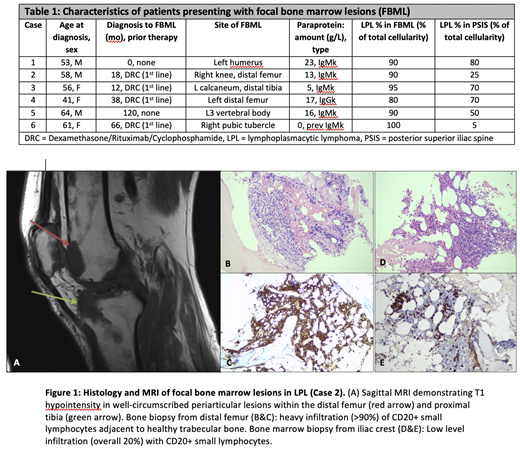
MRI demonstrated well-defined areas of abnormal signal within the medullae of symptomatic bones and no evidence of cortical or trabecular bone involvement (Fig. 1A), with T1 hypointensity, diffusion restriction and mild STIR hyperintensity in excess of background BM changes. The FBML showed a predilection for the lower limbs (knees, ankles and/or feet involvement in 3 patients, 50%) and periarticular spaces (5 patients, 83%). They were CT-occult and only mildly FDG-avid.
In all cases, core biopsies of the FBML showed heavy BM infiltration with diffuse interstitial infiltrate of small, mature lymphocytes (Fig. 1B, 1C) expressing CD20, CD79a and IgM; CD138 staining highlighted scattered interstitial plasma cells (<10%). There was no evidence of infarct or high-grade transformation. When compared to contemporaneous PSIS biopsies (Fig. 1D, 1E), all FBML demonstrated significantly higher proportions of lymphoid infiltration into the haematopoietic space (80-100% vs 0-80%, p=0.03), confirming FBML represent heavier disease infiltrationthan the background BM disease burden.
On blood panels, LDH, ALP and platelet count were normal and Hb was >100g/L in all but the 1 patient with active WM (who had Hb 79g/L and ALP 149IU/L). There was no corresponding rise in paraprotein at the time of FBML presentation.
In all cases, the detection of FBML prompted initiation/escalation of systemic treatment despite no evidence of progression using conventional criteria; this resulted in resolution of pain and disappearance of the T1 hypointense lesions on MRI in all cases.
CONCLUSION
Painful, LPL-dense, infiltrative lesions confined to the BM of affected appendicular bones are an unreported manifestation in WM. They are clinicopathologically different from the osteolytic lesions seen in myeloma and the painless, diffuse BM infiltration seen in low-grade lymphomas and can induce unrelenting focal skeletal pain. Based on our findings, such cases should be evaluated for focal bone marrow lesions; MRI is the preferred modality as lesions are CT-occult. They do not necessarily represent high-grade transformation. This small yet comprehensive series suggests they constitute a hitherto undescribed novel indication for systemic therapy irrespective of the general disease status.
D'Sa:Janssen:Honoraria, Research Funding;BeiGene:Honoraria, Research Funding;Sanofi:Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.