Background
Care coordination can be especially challenging in the setting of rare malignancies such as myelofibrosis (MF), where hematology/oncology teams have limited experience working together to implement rapidly evolving standards of care. In this quality improvement (QI) initiative, we assessed barriers to patient-centered MF care in 3 community oncology systems and conducted team-based audit-feedback (AF) sessions within each system to facilitate improved care coordination.
Methods
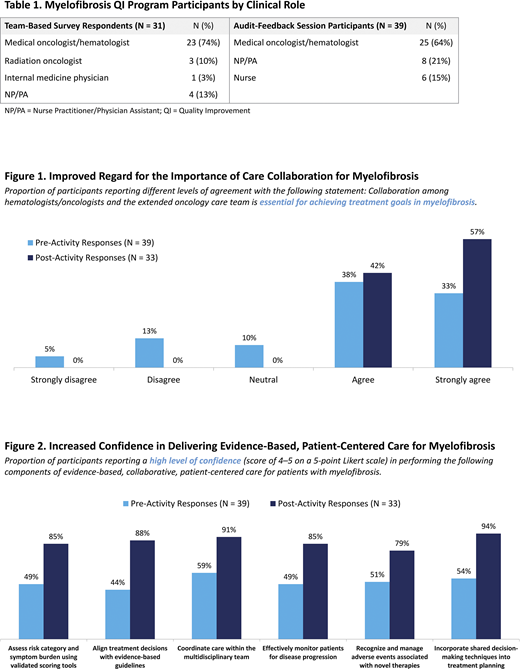
Between 1/2020 and 3/2020, 31 hematology/oncology healthcare professionals (HCPs) completed surveys designed to characterize self-reported practice patterns, challenges, and barriers to collaborative MF care in 3 community oncology systems (Table 1). Building on findings from the team-based surveys, 39 HCPs from these centers participated in AF sessions to reflect on their own practice patterns and to prioritize areas for improved MF care delivery. Participants developed team-based action plans to overcome identified challenges, including barriers to effective risk stratification, care coordination, and shared decision-making (SDM) for patients with MF. Surveys conducted before and after the small-group AF sessions evaluated changes in participants' beliefs and confidence in delivering collaborative, patient-centered MF care.
Results
Team-Based Surveys: HCPs identified managing MF-associated anemia and other disease symptoms (42%), providing individualized care despite highly variable clinical presentations (29%), and developing institutional expertise despite low patient numbers (16%) as the most pressing challenges in MF care. For patients who are candidates for JAK inhibitor therapy, HCPs reported most commonly relying on current guidelines (71%) and clinical evidence (61%) to guide treatment selection. HCPs also considered drug safety/tolerability profiles (55%), personal or institutional experience (13%), and out-of-pocket costs for patients (13%); no participants (0%) reported incorporating patient preference into their decision-making. Teams were underutilizing SDM and patient-centered care resources; fewer than 50% reported providing tools to support adherence (48%), visual aids for patient education (47%), financial toxicity counseling (40%), resources for managing MF-related fatigue (36%), or counseling to reduce risk factors for CVD, bleeding, and thrombosis (26%).
Small-Group AF Sessions: Across the 3 oncology centers, teams participating in the AF sessions (Table 1) shared a self-reported caseload of 97 patients with MF per month. HCPs reported a meaningful shift in beliefs regarding the importance of collaborative care: following the AF sessions, 100% of HCPs agreed or strongly agreed that collaboration across the extended oncology care team is essential for achieving MF treatment goals, an increase from 71% prior to the AF sessions (Figure 1). Participants also reported increased confidence in their ability to perform each of 6 aspects of evidence-based, collaborative, patient-centered care (Figure 2).
In selecting which aspects of patient-centered care to address with their clinical teams, HCPs most commonly prioritized individualizing treatment decision-making based on patient- and disease-related factors (57%), followed by providing adequate patient education about treatment options and potential side effects (24%) and engaging patients in SDM (18%). To achieve these goals, 73% of HCPs committed to sharing their action plans with additional clinical team members; others committed to creating a quality task force to oversee action-plan implementation (15%) and securing buy-in from leadership and stakeholders (9%).
Conclusions
As a result of participating in this community-based QI initiative, hematology/oncology HCPs demonstrated increased confidence in their ability to deliver patient-centered MF care and improved commitment to team-based collaboration. Remaining practice gaps and challenges can inform future QI programs.
Study Sponsor Statement
The study reported in this abstract was funded by an independent educational grant from Incyte Corporation. The grantors had no role in the study design, execution, analysis, or reporting.
Verstovsek:ItalPharma: Research Funding; CTI Biopharma Corp: Research Funding; Promedior: Research Funding; Gilead: Research Funding; NS Pharma: Research Funding; Celgene: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Genentech: Research Funding; Sierra Oncology: Consultancy, Research Funding; PharmaEssentia: Research Funding; AstraZeneca: Research Funding; Incyte Corporation: Consultancy, Research Funding; Blueprint Medicines Corp: Research Funding; Protagonist Therapeutics: Research Funding; Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.