Leukemic blasts and stem cells in AML express high levels of CD123 compared to normal hematopoietic stem and progenitor cells, making CD123 a potential therapeutic target (Kovtun, Blood advances, 2018). IMGN632, a novel CD123-targeting ADC with a humanized IgG1 antibody with high affinity to CD123 and with a DNA single stranded break inducing alkylating payload, demonstrated clinical activity as a monotherapy in patients with relapsed/refractory AML and BPDCN (NCT03386513). We previously reported that the combination of IMGN632 with venetoclax (VEN, a BCL-2 inhibitor) induced additive or synergistic anti-leukemia effects in AML cell lines (EHA 2019, abstract #PF201). Given the FDA approval of the venetoclax and azacitidine (AZA, a hypomethylating agent) combination establishing this regimen as standard-of-care for elderly unfit AML patients, we further characterized the efficacy of the triple combination of IMGN632, VEN and AZA in AML models in vitro and in vivo.
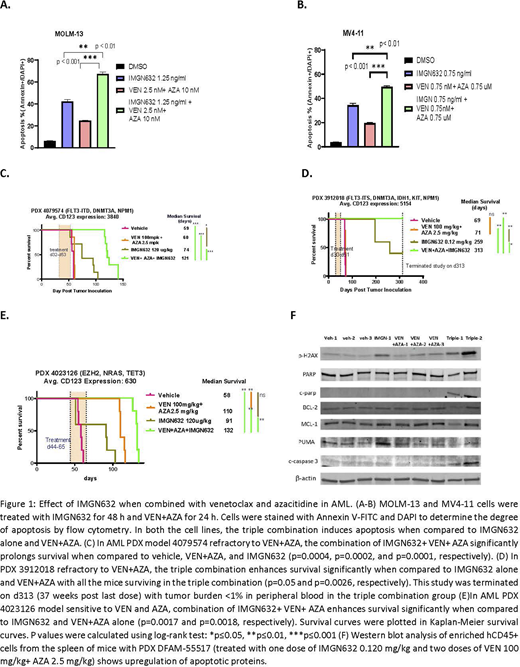
We first tested the cytotoxic effects of IMGN632 alone, VEN+AZA and the triple combination of IMGN632+VEN+AZA in AML cells lines. MOLM-13 and MV4-11 cells treated with varying doses of IMGN632 for 48 hours and VEN + AZA for 24 hours, reduced cell viability in a concentration- dependent manner. Treatment with the triple combination induced greater apoptosis and increased cell death when compared to IMGN632 alone or VEN+AZA, with the triple combination demonstrating synergism (CI=0.26) (Fig A-B). This was supported by western blot analysis in which MOLM-13 cells showed upregulation of apoptotic proteins such as cleaved-PARP, PARP, cleaved-caspase 3 and DNA damage markers, p-H2AX and ICAD in the triple combination.
We next tested the efficacy of the triple combination of IMGN632, VEN and AZA in four AML PDX models. We injected AML PDX cells into NSG mice and upon engraftment in peripheral blood, randomized mice into four cohorts to receive vehicle, IMGN632 (0.120 mg/kg once a week x 3 weeks), VEN (100 mg/kg, 5 days/week x 3 weeks) with AZA (2.5 mg/kg, daily x 5 days) or the triple combination. The endpoints included circulating leukemia burden monitored by hCD45/hCD123 flow cytometry of serial peripheral blood (PB) samples and mice survival.
WIMGN632 alone reduced leukemia burden and extended survival in all 4 CD123+ PDX models tested (median overall survival of 131 ± 74 days (range, 74-259) for IMGN632 vs. 59 ± 7 days (range 49-69) for vehicle). The triple combination showed superior anti-leukemic efficacy in all four PDX models, compared to IMGN632 or VEN+AZA alone (triple combination median overall survival of 152 days ± 99 days (range 41-313) vs. 131±74 days in IMGN632 (range 74-259) and 73 ± 22 days (range 51-110) in VEN+AZA. In two models refractory to VEN+AZA, triple therapy significantly exceeded efficacy of IMGN632 monotherapy, indicating synergy and potential to overcome VEN+AZA resistance with the triplet; no tumor cells were detected in the triple-treated cohort in PDX 3912018 when the experiment was terminated at day 313 (Fig C and D) . In one model sensitive to VEN+AZA, the triple combination showed superior anti-leukemic efficacy compared to VEN+AZA or IMGN632 monotherapy (Fig E).
In DFAM-55517 PDX model, western blot analysis of enriched hCD45+ cells from the spleen of mice collected 48 hours after the administration of one dose of IMGN632 and two doses of VEN+AZA (n=3/group) showed an upregulation of apoptotic proteins, cleaved-PARP and cleaved caspase-3, and upregulation of the DNA damage marker p-H2AX in the triple combination group (Fig. F).
In summary, combining IMGN632 with VEN and AZA induced synergistic cell death in AML cell lines and significantly improved survival in multiple AML PDX models compared with AZA+VEN doublet or IMGN632 monotherapy. These data support the addition of a CD123-targeted ADC with a novel DNA-damaging payload to the standard of care, AZA+VEN in AML patients. The combination of IMGN632 and Venetoclax and/or Azacitidine is being tested in an ongoing Phase Ib/II clinical trial (NCT04086264).
Daver:Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Servier: Research Funding. Sloss:ImmunoGen, Inc.: Current Employment. Zweidler-McKay:ImmunoGen, Inc.: Current Employment. Konopleva:Cellectis: Research Funding; Reata Pharmaceutical Inc.;: Patents & Royalties: patents and royalties with patent US 7,795,305 B2 on CDDO-compounds and combination therapies, licensed to Reata Pharmaceutical; Stemline Therapeutics: Consultancy, Research Funding; Eli Lilly: Research Funding; Sanofi: Research Funding; Genentech: Consultancy, Research Funding; Kisoji: Consultancy; AstraZeneca: Research Funding; F. Hoffmann La-Roche: Consultancy, Research Funding; Agios: Research Funding; Ablynx: Research Funding; Amgen: Consultancy; Forty-Seven: Consultancy, Research Funding; Calithera: Research Funding; AbbVie: Consultancy, Research Funding; Rafael Pharmaceutical: Research Funding; Ascentage: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.