Introduction
Eptacog beta [Sevenfact®, coagulation factor VIIa (recombinant)-jncw] (HEMA Biologics and LFB) is a human rFVIIa variant indicated for the treatment and control of bleeding events (BEs) in adults and adolescents with hemophilia A or B with inhibitors. Eptacog beta has not yet been approved for perioperative management; therefore, a phase 3 trial (PERSEPT 3, NCT02548143) was initiated to evaluate the safety and efficacy of eptacog beta for the prevention of excessive bleeding and achievement of hemostasis in persons with hemophilia A or B with inhibitors (PwHABI) undergoing elective surgery or other invasive procedures.
Aims
To determine the perioperative safety and efficacy of eptacog beta in major and minor elective procedures in PwHABI.
Methods
PERSEPT 3 was a global, multicenter, single-arm, phase 3 trial that enrolled male PwHABI who required elective minor or major procedures. IRB approval was obtained, and all subjects provided informed consent.
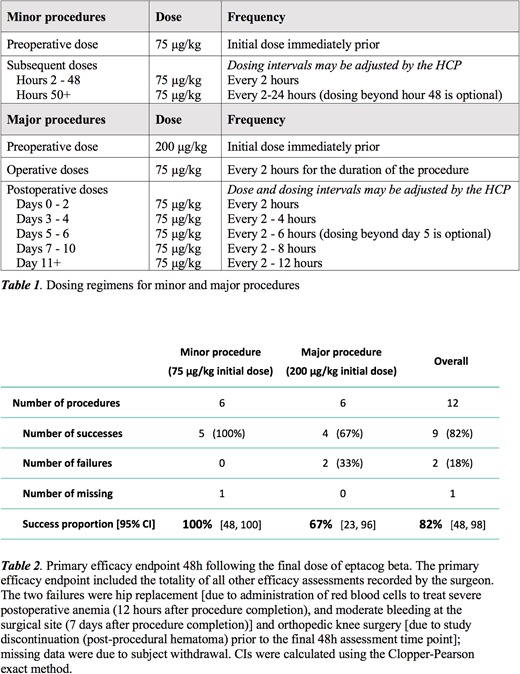
Immediately prior to the start of the procedure, subjects undergoing minor invasive procedures were administered an initial dose of 75 µg/kg eptacog beta and those undergoing major invasive procedures were administered an initial dose of 200 µg/kg eptacog beta. Additional eptacog beta (75 µg/kg) was administered during the procedure and post-operatively (Table 1). Hemostatic efficacy was assessed using a 4-point evaluation scale during the procedure, immediately following the procedure, at regular post-operative intervals, and 48 hours following the last dose of eptacog beta (hemostatic evaluations were recorded as excellent, good, moderate and poor). The primary efficacy endpoint was the percentage of good and excellent responses (ie, successes) at 48 (±4) h following the final dose of eptacog beta and was based upon the investigators' integrated assessment; taking into consideration the investigators' intraoperative hemostatic assessment; the number of postoperative bleeding events and interventions, and blood transfusions; and the amount of eptacog beta used.
Results
Twelve male subjects (age 2-56 years; median 20 years) were enrolled at 8 sites in 5 countries; all subjects had severe hemophilia A with inhibitors. Six minor procedures [circumcision (3) and tooth extraction (3)] and 6 major procedures (left transtibial amputation, hip replacement, orthopedic knee surgery, amputation of the left leg, left knee joint endoprosthesis removal, and left ankle achilloplasty) were evaluated.
The primary efficacy endpoint is shown in Table 2: the success proportion was 67% for major procedures and 100% for minor procedures. The intraoperative efficacy of eptacog beta was rated as good or excellent in all 12 procedures (100% success); mean estimated actual intraoperative blood loss was lower than the mean maximum predicted blood loss for a patient without a bleeding disorder undergoing the same procedure. Efficacy 24 hours following procedure completion was rated as good or excellent in all procedures where data were reported (major, 4/4; minor, 6/6).
One subject (major procedure) was withdrawn from the study due to an adverse event (postprocedural hematoma); this subject subsequently received aPCC and NSAIDs and experienced blood loss anemia and GI hemorrhage. One subject (minor procedure) withdrew consent. Other nontreatment-related adverse events included postoperative anemia, post-procedural hemorrhage, procedural pain, wound secretion and hemorrhage. No allergic, hypersensitivity, or anaphylactic events were reported; no anti-eptacog beta antibodies were observed; and no thromboembolic events occurred.
Conclusions
This controlled study demonstrated the efficacy and safety of 2 different dose regimens of eptacog beta in minor and major elective procedures. The intraoperative hemostatic efficacy of eptacog beta was rated as good or excellent in all major and minor procedures, and the success proportion 48h following the final dose (primary efficacy endpoint) was 82% overall. The breadth of procedures examined (ranging from circumcision and tooth extraction to amputation and hip replacement), low mean blood loss, and high intraoperative efficacy suggests that eptacog beta may be successfully utilized in a variety of minor and major procedures. This study provides useful perioperative data for providers who manage congenital hemophilia A or B patients with inhibitors.
Escobar:National Hemophilia Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees. Quon:Genentech, Inc./F. Hoffmann-La Roche Ltd: Honoraria, Speakers Bureau; Novo Nordisk: Honoraria, Speakers Bureau; Shire/Takeda: Speakers Bureau; Octapharma: Honoraria; Bayer: Honoraria; Biomarin: Honoraria, Speakers Bureau; Orthopaedic Institute for Children: Current Employment; Bioverativ/Sanofi: Honoraria, Speakers Bureau. Leissinger:Bayer: Consultancy; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy; HEMA Biologics: Consultancy; Takeda: Consultancy; Uniqure: Consultancy; Kedrion: Consultancy; Spark: Consultancy. Luck:Orthopaedic Institute for Children: Research Funding; National Hemophilia Foundaton: Honoraria, Speakers Bureau; Board of Trustees, Orthopaedic Institute for Children: Membership on an entity's Board of Directors or advisory committees. Al-Sabbagh:LFB: Current Employment. Bonzo:LFB USA, Inc.: Current Employment; American Statistical Association: Other; International Statistics Institute: Other; International Association for Statistical Computing: Other. Mitchell:HEMA Biologics: Consultancy. Alexander:HEMA Biologics, LLC: Current Employment, Patents & Royalties: No royalties or benefits. Hermans:Bayer: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding, Speakers Bureau; Shire, a Takeda company: Consultancy, Research Funding, Speakers Bureau; Sobi: Consultancy, Research Funding, Speakers Bureau; Biogen: Consultancy, Speakers Bureau; CAF-DCF: Consultancy, Speakers Bureau; CSL Behring: Consultancy, Speakers Bureau; LFB: Consultancy, Speakers Bureau; Novo Nordisk: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Octapharma: Consultancy, Speakers Bureau; Kedrion: Speakers Bureau; EAHAD: Other; WFH: Other.
SEVENFACT(R) [eptacog beta, coagulation factor VIIa (recombinant)-jncw] is a human rFVIIa variant indicated for the treatment and control of bleeding events (BEs) in adults and adolescents with hemophilia A or B with inhibitors. Eptacog beta is not yet indicated for perioperative management
Author notes
Asterisk with author names denotes non-ASH members.