Background: Blinatumomab is approved for the treatment of adult patients (pts) with relapsed/refractory Philadelphia chromosome-negative B-cell precursor acute lymphoblastic leukaemia (R/R Ph- BCP-ALL). We present interim 3-year results of a multi-country observational study in R/R Ph- BCP-ALL pts, including the subset with late first relapse (LFR, defined as first remission duration of ≥12 months [mos]). LFR pts were excluded from the pivotal studies of R/R Ph- BCP-ALL and data is limited on blinatumomab treatment outcomes in this population.
Methods: This study included adult pts who initiated blinatumomab between 22 March 2017 and 12 February 2020 in routine clinical practice. Pts who received blinatumomab in a clinical trial or an expanded access program were excluded. Informed consent was sought according to local guidelines. Data was extracted from medical records: pts were categorized as LFR by the treating clinicians. Pts were followed-up until data cut-off date, death, loss-to-follow-up, or withdrawal: whichever came first. Denominators of percentages excluded pts with missing data unless indicated. Kaplan-Meier (KM) estimates were calculated to investigate survival outcomes at 24 mos: relapse-free survival (RFS) defined as the interval between complete remission with full/partial/incomplete recovery of peripheral blood counts (CR/CRh/CRi) and relapse (bone marrow blasts >5%) or death; and overall survival (OS) defined as the interval from blinatumomab initiation to death. Cumulative Incidence Function (CIF) estimates were calculated for: mortality (independent of relapse) following allogeneic haematologic stem cell transplant (alloHSCT), and relapse (independent of unrelated death) after alloHSCT.
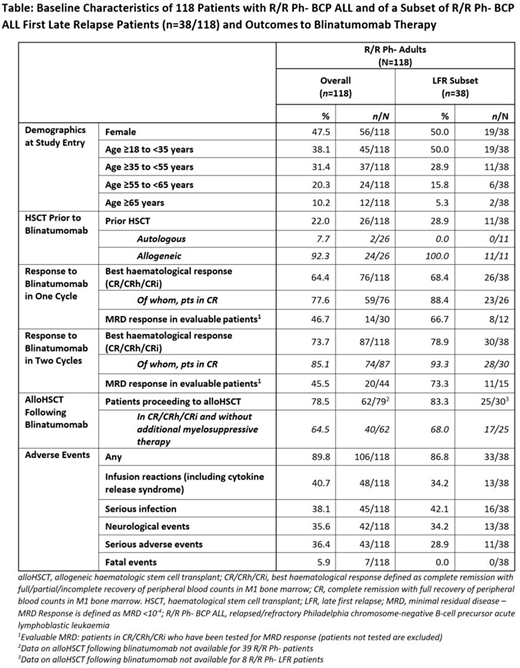
Results: A total of 118 R/R Ph- pts were included: median age was 45.5 years (interquartile range [IQR]: 29.0, 58.0) with 47.5% (n=56) being female. For the subset of R/R Ph- pts with LFR (n=38), median age was 34.5 years (IQR: 23.0, 50.0) with 50.0% being female. Among all pts: 22% (n=26) had previous HSCT (28.9% [n=11] in the LFR subset), 100% (n=118) were treated with ≥1 prior anti-cancer therapy, 42.4% (n=50) underwent first salvage, 8.5% (n=10) second salvage, and 0.8% (n=1) third salvage.
Within 2 blinatumomab cycles, 73.7% (n=87) R/R Ph- pts achieved CR/CRh/CRi: among CR/CRh/CRi pts with evaluable minimal residual disease (MRD, n=44), 45.5% had MRD response (Table). The majority (78.5%, n=62) of pts proceeded to alloHSCT, of whom 64.5% (n=40) had achieved CR/CRh/CRi and had no additional myelosuppressive therapy (Table). The KM estimates for RFS and OS at 24 mos were 50.0% (95% confidence interval [CI]: 37.0, 62.0) and 58.0% (95% CI: 46.0, 68.0), respectively. In pts who achieved CR/CRh/CRi and had no additional therapy before proceeding to alloHSCT, the CIF estimates for relapse and mortality at 1 year following alloHSCT were 8.0% (95% CI: 2.0, 20.0) and 13.0% (95% CI: 5.0, 26.0) respectively.
In the LFR subset, 78.9% (n=30) achieved CR/CRh/CRi within 2 cycles: among evaluable pts (n=15) 73.3% (n=11) had MRD response (Table). Most pts (83.3%, n=25) proceeded to alloHSCT, of whom 77.3% (n=17) had achieved CR/CRh/CRi and had no additional myelosuppressive therapy (Table). At 24 mos, the KM estimates for RFS and OS were 70.0% (95% CI: 47.0, 85.0) and 74.0% (95% CI: 51.0, 87.0), respectively. In pts who achieved CR and proceeded to alloHSCT without other therapy, the CIF estimates for relapse and mortality at 1 year were both 7.0% (95% CI: 0.0, 27.0).
Among R/R Ph- pts, 89.8% (n=106) had adverse events (AEs) occurring between blinatumomab initiation and ≤30 days following the final infusion, 36.4% (n=43) reported serious AEs (SAEs) and 5.9% (n=7) fatal events: 1 fatal AE was blinatumomab-related. AEs were reported in 86.8% of LFR pts (n=33), and 28.9% (n=11) of pts experienced an SAE (no fatal events occurred). In both subgroups, the most commonly reported AEs (in >34% of pts) were infusion reactions (including cytokine release syndrome), serious infections, and neurological events (Table).
Conclusion: In real-world clinical practice, blinatumomab was an effective therapy in pts with R/R Ph- ALL including those with LFR. Almost three quarters of pts achieved CR/CRh/CRi within two cycles. Most pts proceeded to alloHSCT, the majority in CR/CRh/CRi and without additional myelosuppressive therapy. The safety data are consistent with the established safety profile of blinatumomab.
Rijneveld:Servier: Research Funding; Amgen: Research Funding. Fracchiolla:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Speakers Bureau; ABBVIE: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses. Šálek:Amgen: Consultancy, Honoraria, Research Funding. Chiaretti:Amgen: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees. Alam:Amgen: Current Employment, Current equity holder in publicly-traded company. Pezzani Grueter:Amgen: Current Employment, Current equity holder in publicly-traded company, Other: Travel, accommodations, expenses. Mohammad:Amgen: Current Employment, Current equity holder in publicly-traded company. Kormany:Amgen: Current Employment, Current equity holder in publicly-traded company. Kreuzbauer:Amgen: Current Employment, Current equity holder in publicly-traded company. Rambaldi:Omeros: Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Research grant from Amgen Inc.; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Advisory board and speaker fees from Pfizer.; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Astellas: Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support of parent study and funding of editorial support. Received travel support., Research Funding; BMS/Celgene: Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); University of Milan: Current Employment; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support from Gilead.; Sanofi: Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company).
Author notes
Asterisk with author names denotes non-ASH members.