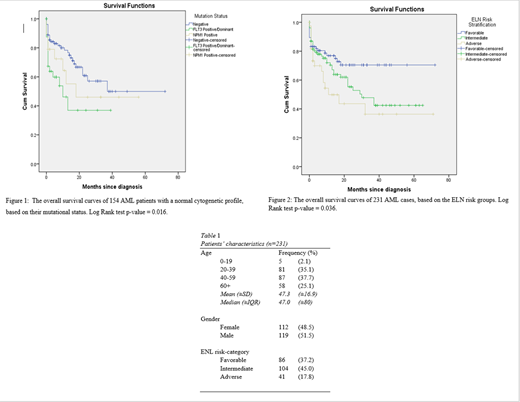
Introduction: AML is a malignant clonal disease that affects mainly the elderly [1]. Data on younger populations, particularly from the Middle East is scarce. The aim of this retrospective study is to investigate the risk factors that are associated with lower survival, particularly when applying the European Leukemia Network (ELN) prognostic scheme [2].Methods: Overall survival (OS) was calculated from the date of diagnosis until the date of death from any cause. Kaplan-Meier curves were plotted to evaluate OS [3]. Log Rank test was used to assess the statistical significance of our findings. Results: This study included 231 AML cases, with a median age of 47 years, ranging from 16 to 96 years of age. The majority of the cases are young (20-39: 35.1%; 40-59: 37.7%). Females accounted for 48.5% of all cases (table 1). Cases were assigned to a risk group based on the ELN risk stratification scheme (table 1). Accordingly, 86 (37.2%) cases were assigned to the favorable group, 104 (45.0%) patients were in the intermediate group, and 41 (17.8%) enrollees were in the adverse group. Among the 154 cases with a normal cytogenetic profile, 32 cases were FLT3 positive/dominant, 19 patients tested positive for NPM1 only, and 103 enrollees were negative for both mutations. Figure 1 shows the negative impact of a positive/dominant FLT3 mutational status on OS, with a median survival time of 10 months (95% CI: 1.44 - 18.56). The other groups have not reached their median survival. Figure 2 illustrates that those who are among the ELN adverse group fared the worst in terms of OS, with a median survival of 11 months (95% CI: 0.00-22.31). Additionally, the median survival of the intermediate risk groups is 30 months (95% CI: 14.61 - 45.39). The favorable risk group has not reached its median survival. Conclusion: This retrospective study confirms the impact of the ELN risk categories, and the detrimental effects of FLT3 mutation on OS among AML cases. Currently, FLT3 inhibitors have been shown to improve leukemia free survival in those who are mutated at presentation, as well as at the time of relapse [4, 5]. Alternative strategies that are less toxic but more effective are needed to improve the survival of older patients. ELN risk-grouping is robust in our cohort of younger cases , and can be used to guide the choice of intensive treatment, particularly for bone marrow transplantation or enrollment into clinical trials.
References
1. Arber, D.A., et al.,The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia.Blood, 2016.127(20): p. 2391-405.
2. Döhner, H., et al.,Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel.Blood, 2017.129(4): p. 424-447.
3. Altman, D.G. and J.M. Bland,Time to event (survival) data.Bmj, 1998.317(7156): p. 468-469.
4. Perl, A.E., et al.,Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML.New England Journal of Medicine, 2019.381(18): p. 1728-1740.
5. Levis, M.,Midostaurin approved for FLT3-mutated AML.Blood, The Journal of the American Society of Hematology, 2017.129(26): p. 3403-3406.
Pandita:Novartis:Honoraria;Pfizer:Honoraria;Amgen:Honoraria;AstraZeneca:Honoraria;Takeda:Honoraria;Jansen:Honoraria;Cilag:Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.