Background: The tumor microenvironment (TME) of MM is characterized by an immunosuppressive milieu fostering MM growth. The impact of tumor associated macrophages (TAM) has not been well-studied in the MM TME. We evaluated the bone marrow biopsies (BMBx) obtained at the initial diagnosis of MM and evaluated the correlation between the number of CD68+ macrophages and clinical parameters and outcomes.
Methods: We evaluated the BMBx at initial diagnosis of 34 consecutive MM patients (pts) diagnosed between 2007-2016 at Mayo Clinic Florida. Immunohistochemistry (IHC) was performed with an anti-CD68 (KP1) antibody to identify and count CD68+ TAMs. Survival was analyzed with the Kaplan-Meier method. Chi-square tests were performed to study the correlation between the number of CD68+ TAMs and various clinical parameters.
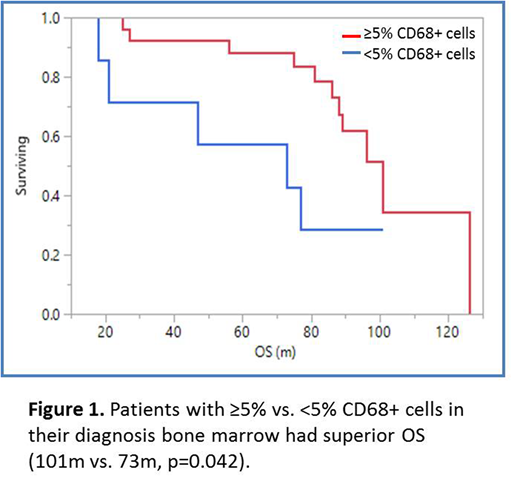
Results: Nineteen (56%) pts were male, 29 (85%) were caucasian. Median age at MM diagnosis was 58.5 years (95% CI 57-63), 18 (53%) pts has ISS I/II disease, 13 (38%) pts had ISS III disease, 3 (9%) has unknown ISS. Eight (24%) had high risk cytogenetics; 4(12%) had del17p, 2 (6%) had t(14;16), 1(3%) had 1q amplification, 3 (9%) had t(4;14). Eight (24%) had t(11;14). Twenty nine (85%) pts underwent autologous transplantation, 11(32%) received maintenance therapy. Overall response rate (≥partial response) to induction therapy was 100%. The ≥ very good partial response (VGPR) rate was 73.5%. Median number of induction cycles was 4.5 (95% CI 4.8-8.3). Twelve (35%) pts received lenalidomide (R)-dexamethasone (D) induction (RD), 9 (26%) received bortezomib (V)-cyclophosphamide(C)-D (VCD) induction, 7 (21%) received VRD induction, 5(15%) received VD induction and 1(3%) received CRD induction. The median follow-up was 92 months (m) (95% CI 85-99) The median progression free survival (mPFS) of the pt population was 40m (95% CI 27-68) and the median overall survival (mOS) was 96m (95% CI 81-126). Mean CD68+ TAM count was 12.5% (95% CI 8.1-16). There was a trend towards improved PFS for patients with ≥5% vs. <5% CD68+ TAM (47m vs. 36m, p=0.055) and there was improved OS for patients with ≥5% vs. <5% CD68+ TAM (101m vs. 73m, p=0.042, Figure 1) in their initial diagnostic BMBx. Pts with high risk cytogenetics did not have a significantly different CD68+ TAM (8.6%vs.12.8%, p=0.363) compared to those with standard risk cytogenetics. Pts with ISS I/II disease were more likely to have ≥5% CD68+ TAM in their diagnosis BMBx compared to patients with ISS III disease, χ²=8.58, p=0.003. Pts who achieved ≥VGPR to induction therapy were more likely to have ≥5% CD68+ TAM in their diagnosis BMBx compared to those who achieved <VGPR; χ²=5.35, p=0.020. Pts who received R-based induction and had ≥5% CD68+ TAM in their diagnosis BMBx had superior OS (96m vs. 62m, p=0.044) compared to those with <5%.This finding was not seen in patients who received V-based induction (p=0.33).
Conclusion: Pts with ≥5% CD68+ TAM in their initial diagnostic BMBx are more likely to have deeper responses to induction therapy as well as superior OS. In pts induced with an R-based regimen, increased CD68+ TAM was associated with significantly better OS raising the possibility of a therapeutic and mechanistic role of TAM in R-related therapeutic effect. ≥5% CD68+ TAM were noted in patients with lower risk disease as defined by ISS stage but not by cytogenetics. Further exploration of the role of TAM in the biology of MM and its impact on clinical and therapeutic outcomes is warranted.
Alhaj Moustafa:Acrotech: Consultancy. Tun:DTRM Biopharma: Research Funding; Acrotech: Research Funding; Bristol-Myers Squibb: Research Funding; Celgene: Research Funding; Mundipharma: Research Funding; Curis: Research Funding; TG Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.