Introduction: AML is a heterogeneous disease of mainly older pts, with a median age of 68 y at diagnosis. Previous studies have shown that age is one of the most important prognosticators for overall survival (OS) of AML pts. In addition, several cytogenetic and molecular markers have been used for risk stratification of AML pts and to guide therapeutic decisions. However, the role of these established molecular marker (e.g. FLT3-ITD and NPM1 mutations) in pts aged ≥70 y is not fully understood.
Aims: The goal of this study was to analyze the prognostic role of FLT3-ITD and other molecular markers in AML pts ≥70 y. We also compared this cohort with pts 60-69 y, who received a similar treatment, to identify potential differences between these age groups.
Methods: We analyzed 269 de novo AML pts ≥70 y and 250 pts 60-69 y. All pts were treated on frontline Cancer and Leukemia Group B/Alliance for Clinical Trials in Oncology protocols that were based on standard intensity cytarabine/anthracycline induction followed by consolidation with varied chemotherapy regimens. None of the pts received an allogeneic stem cell transplant in first complete remission (CR). In pretreatment bone marrow or blood samples, the mutational status of 80 cancer- and leukemia-associated genes (Eisfeld et al., Leukemia 2017;31:2211) were determined using a targeted next-generation sequencing panel. All variants that occurred with variant allele fractions of <0.1 were defined as not mutated. CEBPA mutations were determined using Sanger sequencing, and the presence of FLT3-ITD using fragment analysis (cut-off >.05). The pts' pretreatment karyotypes were determined locally and reviewed centrally.
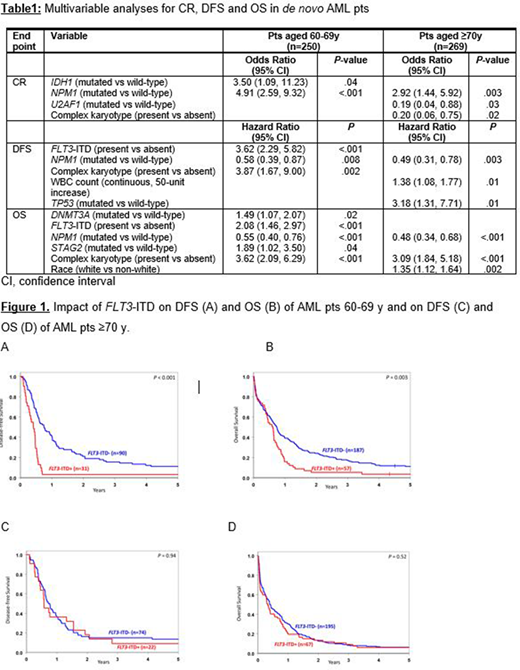
Results: There were no significant differences in frequencies of any clinical or specific cytogenetic markers between AML pts ≥70 y and pts 60-69 y. Pts 60-69 y presented more often with Favorable-risk disease based on the European LeukemiaNet classification (Döhner et al. Blood 2017;129:424; 30% vs 23%, P<.001), whereas pts ≥70 y were more often classified as Adverse-risk (45% vs 51%, P<.001). Concerning recurrent gene mutations, we found that pts ≥70 y less frequently harbored mutations in NPM1 (28% vs 44%; P<.001) and PTPN11 (4% vs 8%; P=.03), and more frequently mutations in RUNX1 (21% vs 10%; P<.001) than pts 60-69 y. In line with previous studies, outcome for both groups of older pts was generally poor. Pts 60-69 y had a higher CR rate (50% vs 37%, P=.003) and longer overall survival (OS; median: 0.7 y vs 0.4 y, P=.003) compared with pts ≥70 y. There was no significant difference in disease-free survival (DFS) between the age groups. Next, we analyzed the prognostic role of FLT3-ITD (present in 26% of pts ≥70 y and 23% of pts 60-69 y) and found that its presence had a prognostic impact on DFS (P<.001) and OS (P=.003) only in pts 60-69 y but not in pts ≥70 y in univariate analyses (Figure 1) and in the final multivariable analyses (MVA, Table 1). In these pts, FLT3-ITD was found to be associated with worse outcome independent from the FLT3-ITD allelic ratio or the presence of additional NPM1 mutations. Similarly, to FLT3-ITD, DNMT3A mutations associated with shorter OS only in pts 60-69 y in MVA. In the final MVA for both pts 60-69 y and those ≥70 y, NPM1 mutations were the only prognostic marker associated with better outcome for all clinical endpoints tested (i.e., CR rates, DFS and OS) (Table 1), while complex karyotype (i.e., the presence of ≥3 chromosome aberrations) was a poor prognostic factor both in pts 60-69 y (DFS, OS) and those ≥70 y (CR rates, OS). In contrast, in MVA of pts ≥70 y, mutations in U2AF1 associated with lower CR attainment (P=.03), and TP53 mutations with shorter DFS (P=.01).
Conclusions: Although pts aged ≥70 y had similar pretreatment clinical and cytogenetic features as pts 60-69 y, we showed that distinct differences can be found regarding prognosticators. Interestingly, we found that presence of a FLT3-ITD had no impact on outcome of pts ≥70 y, regardless of allelic ratio or presence of an additional NPM1 mutation. Only NPM1 mutations and complex karyotype had a prognostic impact on both age groups.
Support: U10CA180821, U10CA180882 U24CA196171; https://acknowledgments.alliancefound.org; Clinicaltrials.gov Identifiers: NCT00048958 (CALGB-8461), NCT00899223 (CALGB 9665), and NCT00900224 (CALGB 20202)
Walker:Vigeo Therapeutics: Consultancy; Karyopharm: Current Employment, Current equity holder in publicly-traded company. Blachly:AbbVie, AstraZeneca, KITE Pharma: Consultancy. Wang:Bristol Meyers Squibb (Celgene): Consultancy; Astellas: Consultancy; Jazz Pharmaceuticals: Consultancy; Macrogenics: Consultancy; PTC Therapeutics: Consultancy; Abbvie: Consultancy; Stemline: Speakers Bureau; Pfizer: Speakers Bureau; Genentech: Consultancy. Kolitz:Pfizer: Membership on an entity's Board of Directors or advisory committees; Magellan: Membership on an entity's Board of Directors or advisory committees. Powell:Rafael Pharmaceuticals: Consultancy, Other: Advisor, Research Funding; Pfizer: Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Advisor, Research Funding; Novartis: Research Funding; Genentech: Research Funding. Stone:Gemoab: Consultancy; Syros: Consultancy; Arog: Consultancy, Research Funding; Celgene: Consultancy, Other; Macrogenics: Consultancy; Agios: Consultancy, Research Funding; Janssen: Consultancy; Aztra-Zeneca: Consultancy; Biolinerx: Consultancy; Trovagene: Consultancy; Daiichi-Sankyo: Consultancy; Argenix: Other; Actinium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Other: DSMB; Abbvie: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Astellas: Consultancy; Syntrix: Other: DSMB; Jazz: Consultancy; Stemline: Consultancy; Pfizer: Consultancy; Syndax: Consultancy, Research Funding. Byrd:Novartis: Research Funding; Acerta Pharma: Research Funding; Syndax: Research Funding; Pharmacyclics LLC, an AbbVie Company, Janssen, Novartis, Gilead, TG Therapeutics: Other; Vincera: Research Funding; Kartos Therapeutics: Research Funding; Trillium: Research Funding; Pharmacyclics LLC, an AbbVie Company, Gilead, TG Therapeutics, BeiGene: Research Funding; Pharmacyclics LLC, an AbbVie Company, Gilead, TG Therapeutics, Novartis, Janssen: Speakers Bureau; Leukemia and Lymphoma Society: Other; Janssen: Consultancy. Eisfeld:Vigeo Therapeutics: Consultancy; Karyopharm: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.