Introduction
Increased susceptibility to infections in MDS is due to neutropenia as well as functional impairment of neutrophils. We investigated whether neutrophil dysfunction includes diminished formation of neutrophil extracellular traps (NETs). The latter are networks of extracellular fibers, primarily composed of DNA, which bind pathogens. This component of the immune system's first line of defense was first described in 2004 by Zychlinsky and coworkers. Formation of NETS is triggered by reactive oxygen species (ROS), which in neutrophils are mainly produced by NADPH-oxidase and myeloperoxidase (MPO).
Methods
Fluorescence microscopy was used to show NET formation in patients with MDS (n=12) (1 MDS del(5q), 1 MDS-RS-SLD, 1 MDS-RS-MLD, 5 MDS-MLD, 2 MDS-EB-1, 2 MDS-EB-2) in comparison to an age-adjusted normal control group (n=15). Neutrophils were isolated from peripheral blood by density gradient centifugation, sedimented on cover slips in 24-well plates, and incubated with phorbol-12-myristate-13-acetate (PMA) for 30 and 180 minutes to stimulate NET formation. Neutrophil elastase was detected by primary anti-NE (Abcam) plus secondary goat anti-rabbit Alexa 488 (FITC), histones by primary anti-histone (Millipore) plus secondary goat anti-mouse Alexa 568 (PE), and cell nuclei by DNA staining with DAPI. Fluorescent images were loaded into Image J/FIJI Software (https://imagej.nih.gov/ij/) for automatic cell detection and assessment of the proportion of cells undergoing NET formation, detectable by positive histone staining.
Flow cytometry was used to further characterize NET formation and demonstrate the production of ROS by NADPH-oxidase and MPO. Intracellular MPO staining was positive for 96.7% and 99.1% of neutrophils in controls and MDS patients, respectively. Reactive oxygen species (ROS) were detected using dihydro-rhodamin 123 (DHR) for hydrogen peroxide, hydroethidine (HE) for superoxide anion, and 3`-(p-aminophenyl) fluorescein (APF) and 3`-(hydroxyphenyl) fluorescein (HPF) for hypochloride (HOCl). In order to block certain components of cellular ROS production, the following inhibitors were used: 4-aminobenzoic hydrazide (ABAH) to block MPO; 4-dimethylamino-antipyrine (AP) to block MPO and quench ROS; and diphenylen-iodonium chloride (DPI) to inhibit NADPH oxidase and mitochondrial respiratory complex I. Flow cytometry data were analysed using CellQuest Software (Becton Dickinson) and FCS Express Reader (De Novo Software).
Results
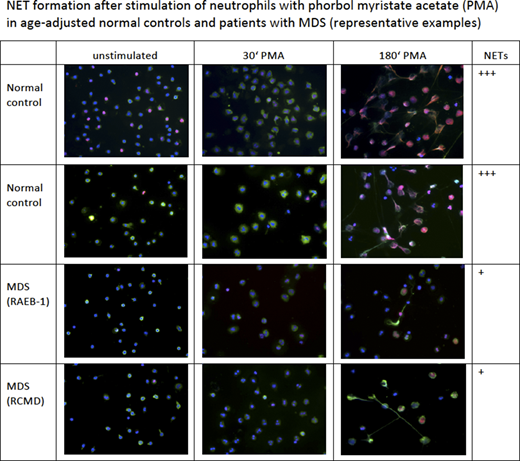
By fluorescence microscopy, marked morphological abnormalities of NET formation were observed. PMA-stimulated neutrophils of patients with MDS generated significantly fewer NETs than neutrophils from controls (increase from 17% to 67% vs. increase from 17% to 85% of cells, respectively) (p=0.02), see Fig. 1. MDS neutrophils showed significantly less cellular swelling after stimulation with PMA (p=0.04). Histone staining showed a more condensed chromatin in MDS neutrophils prior to stimulation (smaller mean fluorescence intensity (MFI) for PE; p=0.05). Upon stimulation with PMA, the decrease in MFI of DAPI, indicating chromatin decondensation, was significantly less in MDS neutrophils than controls (delta MFI 3467 vs. 4687, p=0.03). In addition, assessment of neutrophil elastase showed that its release from cytoplasmic granules (decreasing MFI for FITC) was diminished in patients with MDS (p=0.00002).
On flow cytometry, forward scatter confirmed a significant difference in cell swelling after PMA stimulation (p=0.02). Sideward scatter showed a smaller decrease in granularity after stimulation with H2O2 (p=0.002).
The morphological abnormalities did not seem to be attributable to diminished ROS production since measurement of PMA-stimulated ROS production using HE, and assessing oxidative burst activity using DHR did not reveal significant differences. However, H202-stimulated production of HOCl by MPO was lower in MDS patients, albeit only when MPO activity was simultaneously impeded by inhibitors (ABAH and AP). Inhibition of MPO activity was more easily achieved in patients with MDS (p=0.01), corroborating the notion of a partial MPO defect in MDS.
Conclusions
Our results indicate that NET formation is significantly impaired in MDS neutrophils. Although we found abnormalities of MPO-dependent generation of HOCl, impaired ROS production may not be the only cause of deficient NETosis in MDS.
Gattermann:Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.