Introduction
Selinexor is a first-in-class, oral, potent selective inhibitor of nuclear export (SINE) which blocks XPO1, forcing the nuclear retention and activation of tumor suppressor proteins, leading to cancer cell apoptosis. Selinexor has demonstrated antimyeloma activity in triple class refractory multiple myeloma (MM) [Chari et al. NEJM 2019]. Selinexor synergizes with proteasome inhibitors (PIs) in PI-sensitive and -resistant cell lines and produces high response rates in patients with PI refractory and non-refractory MM. (Bahlis et al. Blood 2018). In the phase 3 BOSTON study, the combination of once weekly (QW) selinexor, QW bortezomib and dexamethasone (SVd) in patients who had received 1-3 prior therapies led to a significantly (47%) longer median progression-free survival (PFS) of 13.93 versus 9.46 months (HR 0.70; P=0.0075) compared to standard twice weekly bortezomib and dexamethasone (Vd). In addition, SVd regimen produced higher response rates and deeper responses (ORR: 76.4% vs 62.3% and ≥CR 16.9% vs 10.2%) compared with Vd. The benefit with SVd was observed across all efficacy endpoints and was associated with lower incidence and severity of bortezomib-induced peripheral neuropathy. Here we present subgroup analyses according to number of prior lines of therapy and prior treatment with lenalidomide (LEN).
Methods
BOSTON is an open-label, randomized phase 3 study in patients with MM comparing SVd (QW selinexor 100 mg, QW subcutaneous bortezomib 1.3 mg/m2, and 20 mg twice weekly [BIW] dexamethasone), versus Vd (1.3 mg/m2 bortezomib BIW and dexamethasone 20 mg 4 x weekly [QIW]). Patients previously treated with a PI must have had at least a partial response (PR) to the PI and 6 months since the last PI regimen. The study primary end point was PFS.
Results
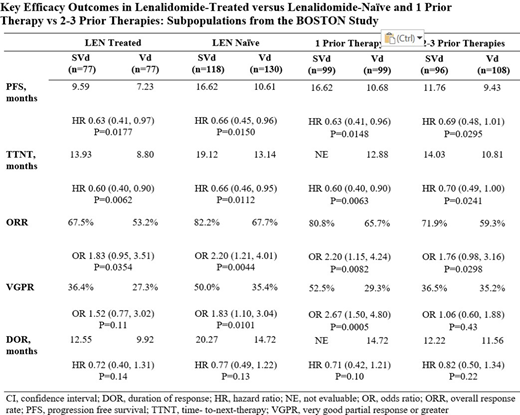
Of the 402 patients in the BOSTON study, 198 (49%) had 1 prior line versus 204 (51%) with 2-3 prior lines. In addition, 154 (38%) comprised the LEN treated subgroup, versus 248 (62%) in the LEN naïve subgroup. Of note, 41% patients received prior thalidomide, which is consistent with most of the BOSTON study patients being treated in the EU. Baseline demographic and disease characteristics were well balanced between treatment arms across subgroups. A very good partial response (VGPR) or better to most recent prior line anti-MM therapy was more frequent in patients with 1 prior line of therapy (63% vs 41%) versus those with 2-3 prior lines of therapy. Patients with prior LEN therapy were more likely to have had 2-3 prior therapies (72.1%) compared with LEN naive patients (37.5%). As shown in the table, SVd was associated with longer PFS compared with Vd in all subgroups. Overall response rates (ORR) and times-to-next-treatment (TTNT) were statistically greater with SVd compared with Vd in all subgroups. Rates of very good partial response or better (VGPR) were statistically greater for SVd vs Vd in the LEN-naïve group and 1 prior treatment group. Grade ≥2 peripheral neuropathy (a key secondary endpoint prespecified in the study) occurred less frequently across all SVd subgroups compared with Vd: LEN treated (21% SVd, 37% Vd, P=0.0166); LEN-naïve (21% SVd, 33% Vd, P=0.0252), 1 prior line (21% SVd, 33% Vd, P=0.0501); 2-3 prior lines (21% SVd, 36% Vd, P=0.0107). Adverse events of ≥grade 3 were more commonly reported in the SVd treatment arm than in the Vd arm, LEN treated (83% SVd, 57% Vd), LEN-naïve (76% SVd, 55% Vd), 1 prior line (77% SVd, 56% Vd), 2-3 prior lines (81% SVd, 56% Vd), and were mostly managed with dose modification and/or supportive treatment. Pneumonia occurred at comparable rates between treatment arms. There were no differences between subgroups in grade 3 adverse events.
Conclusions
In the BOSTON study, once-weekly SVd significantly improved PFS, ORR, TTNT and reduced rates of ≥grade 2 peripheral neuropathy compared with Vd regardless of number of prior treatments or whether patients were previously treated with LEN. Adverse events were managed with dose modification and treatment-related discontinuation rates did not differ between the 2 regimens for any subgroup. The PFS of 16.6 months with SVd after 1 prior therapy, and the HR of 0.63 in patients with prior LEN treatment support the use of once-weekly SVd for the second line treatment of MM following a LEN-containing regimen.
Mateos:Takeda: Honoraria; Abbvie: Honoraria; GlaxoSmithKline: Honoraria; EDO Mundipharma: Honoraria; Seattle Genetics: Honoraria; Roche: Honoraria; Bristol-Myers Squibb: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Adaptive Biotechnologies: Honoraria. Jagannath:Takeda: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Legend Biotech: Consultancy, Honoraria. Delimpasi:GENESIS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Spicka:Celgene, Amgen, Janssen-Cilag, Takeda, Bristol-Myers Squibb, Novartis, Sanofi: Consultancy, Honoraria, Speakers Bureau. Kryachok:Takeda, Janssen, Novartis, Roche, MSD, Bayer: Consultancy, Research Funding; Janssen, Bayer, Karyopharm, MSD, Acerta, AvbbVie, Debiopharm: Research Funding; Takeda, MSD, AbbVie, Ro: Other: Travel, accommodations, expenses; Takeda, Janssen: Consultancy. Gavriatopoulou:Amgen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Genesis Pharma: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Dimopoulos:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees, Research Funding, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees. Auner:Amgen: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Leleu:BMS-celgene: Honoraria; Carsgen: Honoraria; Incyte: Honoraria; Merck: Honoraria; AbbVie: Honoraria; Oncopeptide: Honoraria; Janssen: Honoraria; Novartis: Honoraria; Amgen: Honoraria; GSK: Honoraria; Sanofi: Honoraria; Karyopharm: Honoraria. Sinha:Dr Reddys Lab, Intas Pharmaceuticals, Karyopharm Therapeutics: Honoraria. Venner:Celgene, Amgen: Research Funding; Janssen, BMS/Celgene, Sanofi, Takeda, Amgen: Honoraria. Garg:Janssen, Takeda, Celgene, Novartis, Sanofi: Honoraria. Stevens:Amgen, MorphoSys: Consultancy. Quach:Amgen, Celgene, karyopharm, GSK, Janssen Cilag, Sanofi.: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline, Karyopharm, Amgen, Celgene, Janssen Cilag: Honoraria; GlaxoSmithKline, Karyopharm, Amgen, Celgene, Janssen Cilag: Consultancy; Amgen, sanofi, celgene, Karyopharm, GSK: Research Funding. Moreau:Novartis: Honoraria; Amgen: Consultancy, Honoraria; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Honoraria; Sanofi: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria. Levy:Sanofi: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Bristol Meyers Squibb: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Research Funding, Speakers Bureau; Baylor University Med Center: Current Employment; Takeda: Consultancy, Honoraria, Research Funding. Badros:Amgen: Consultancy; University of Maryland: Current Employment. Anderson:Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding. Bahlis:Genentech: Consultancy, Honoraria; Karyopharm Therapeutics: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; BMS/Celgene and Janssen: Consultancy, Honoraria, Other: Travel, Accomodations, Research Funding; Sanofi: Consultancy, Honoraria. Facon:Celgene, Janssen, Takeda, Amgen, Roche, Karyopharm, Oncopeptides, BMS, Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Cavo:Jannsen, BMS, Celgene, Sanofi, GlaxoSmithKline, Takeda, Amgen, Oncopeptides, AbbVie, Karyopharm, Adaptive: Consultancy, Honoraria. Chai:Karyopharm Therapeutics Inc: Current Employment. Arazy:Karyopharm Therapeutics Inc.: Current Employment. Shah:Karyopharm Therapeutics Inc: Current Employment, Current equity holder in publicly-traded company. Shacham:Karyopharm: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties: (8999996, 9079865, 9714226, PCT/US12/048319, and I574957) on hydrazide containing nuclear transport modulators and uses, and pending patents PCT/US12/048319, 499/2012, PI20102724, and 2012000928) . Kauffman:Karyopharm Therapeutics Inc: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees. Richardson:Celgene/BMS, Oncopeptides, Takeda, Karyopharm: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.