Background: The rapid reduction in tumor burden by the oral B-cell lymphoma-2 inhibitor venetoclax (Ven) poses a risk of tumor lysis syndrome (TLS) for patients (pts) with chronic lymphocytic leukemia (CLL). The approved 5-week Ven dose ramp-up, along with prophylaxis and monitoring, effectively mitigate this risk of TLS but also introduce some logistical challenges to starting the drug. Moreover, the long ramp-up period may lead to clinical deterioration while pts are receiving lower doses, particularly for those with rapid disease progression after B-cell receptor pathway inhibitor (BCRi) therapy. Accelerated Ven dose ramp-up has been explored in a limited number of prior studies, including the prospective M14-032 trial (Jones et al. Lancet Oncol 2018) and a single-center retrospective study (Koenig et al. ASH 2019); these studies had conflicting results with regard to the risk of TLS.
We conducted a multicenter study to systematically characterize the safety profile and feasibility of an accelerated Ven ramp-up schedule in pts with previously treated CLL.
Methods: This is a retrospective chart review from 4 large academic US medical centers of 36 pts with relapsed/refractory CLL and a documented ramp-up period of <5 weeks (duration at treating physician's discretion). The study period was defined from 1 month before to 1 month after ramp-up. TLS risk before Ven initiation was assessed per the label. Safety endpoints included the proportion of pts who experienced Grade ≥3 adverse events (AEs), TLS per Howard criteria (laboratory, clinical, or any reported TLS), and/or metabolic abnormalities observed during ramp-up. Feasibility of accelerated ramp-up was measured by assessing the proportion of pts who reached the target 400-mg dose or an alternative stable dose, defined as continuous/stable Ven dosing for ≥1 month after ramp-up. Descriptive statistics were calculated.
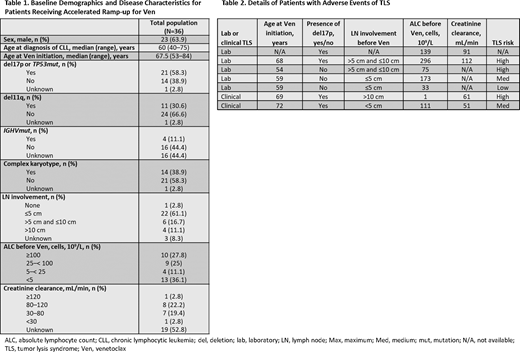
Results: The median age at Ven initiation was 67.5 years (range 53-84) and 58.3% of pts had deletion 17p or TP53 mutation (Table 1). Pts were heavily pretreated (median of 3 lines of prior therapy [range 1-7]) and 88.9% had prior BCRi therapy; 27 pts (75.0%) had BCRi as immediate prior line of therapy before Ven. Before Ven initiation, 7 pts (19.4%) were at high risk of TLS, 13 (36.1%) at medium risk of TLS, and 15 (41.7%) at low risk of TLS; 1 unknown. Last line of therapy was discontinued in 30 pts (83.3%). Twenty-one pts (58.3%) received Ven monotherapy, 11 (30.6%) received Ven plus rituximab, 1 (2.8%) received Ven plus obinutuzumab, and 3 (8.3%) received Ven plus other (including ibrutinib, duvelisib, or idelalisib). Thirty-three pts (91.7%) underwent accelerated ramp-up as an inpatient, with a median hospital stay of 11 days (range 3-28). A stable dose of Ven was reached by 34 pts (94.4%), with median time of 15 days (range 3-66) to stable dose. Twenty-eight of these 34 pts (82.4%) reached the full 400 mg as their stable dose, with median time of 19.5 days (range 11-66). Overall, 15 pts (41.7%) experienced Grade ≥3 AEs and 4 (11.1%) discontinued Ven during ramp-up (2 pts due to clinical TLS, and 1 patient each due to persistent cytopenias and enrollment in hospice care). Seven pts (19.4%) had TLS (5 [13.9%] laboratory, 2 [5.6%] clinical), and 9 pts (25.0%) developed a metabolic abnormality only that did not meet Howard criteria for TLS (Table 2). Both pts with clinical TLS received Ven ramp-up while hospitalized; 1 high risk and 1 medium risk at baseline. Life-threatening (Grade 4) acute kidney injury and hypovolemic shock were reported for both. Of those pts with TLS, 4 (57.1%) required supportive medications. All AEs of TLS (including clinical events) resolved.
Conclusion: In this multicenter study exploring accelerated Ven ramp-up, 82% of pts reached a stable dose of Ven 400 mg. TLS was observed in 19% of pts, including 2 pts with clinical TLS, which in both cases was reversible. Our data suggest that although the risk of TLS with an accelerated ramp-up appears to be greater than with 5-week Ven ramp-up per label, an accelerated Ven ramp-up schedule appears feasible at experienced academic centers with close inpatient monitoring. This dosing regimen may be beneficial for relapsed CLL pts with aggressive disease, particularly those progressing immediately after BCRi therapy. While promising, the approach cannot be routinely recommended until results are validated in a prospective clinical trial.
Davids:BeiGene: Consultancy; AstraZeneca: Consultancy, Research Funding; Adaptive Biotechnologies: Consultancy; Celgene: Consultancy; Janssen: Consultancy; Ascentage Pharma: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Verastem: Consultancy, Research Funding; AbbVie: Consultancy; Eli Lilly: Consultancy; MEI Pharma: Consultancy, Research Funding; Bristol Myers Squibb: Research Funding; Novartis: Consultancy, Research Funding; Sunesis: Consultancy; Gilead Sciences: Consultancy; Zentalis: Consultancy; Syros Pharmaceuticals: Consultancy; Research to Practice: Honoraria; Merck: Consultancy; Surface Oncology: Research Funding. Shadman:TG therapeutics: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Atara Biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cellectar: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mustang Bio: Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Sunesis: Research Funding; Gilead: Research Funding; MophoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sound Biologics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Acerta Pharma: Ended employment in the past 24 months. Parikh:GlaxoSmithKline: Honoraria; AbbVie: Honoraria, Research Funding; Ascentage Pharma: Research Funding; Genentech: Honoraria; Verastem Oncology: Honoraria; Janssen: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; TG Therapeutics: Research Funding; Merck: Research Funding; Pharmacyclics: Honoraria, Research Funding; MorphoSys: Research Funding. Ujjani:MorphoSys: Consultancy; Genentech: Consultancy, Honoraria; Atara: Consultancy, Honoraria; Gilead/Kite: Consultancy, Research Funding; Verastem Oncology: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding. Chen:AbbVie: Other: Institutional research grants, Research Funding. Johnson:AbbVie: Other: Institutional research grants, Research Funding. Jiang:AbbVie: Current Employment, Other: may hold stock or options. Llamas:AbbVie: Current Employment, Other: may hold stock or options. Feng:AbbVie: Current Employment, Other: may hold stock or options. Khan:ICON plc: Current Employment, Other: a company contracted by AbbVie for this study. Lamanna:MingSight: Other: Institutional research grants, Research Funding; Oncternal, Verastem, TG Therapeutics: Other: Institutional research grants, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Columbia University Medical Center: Current Employment; Juno: Other: Institutional research grants, Research Funding; Octapharma: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional research grants, Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bei-Gene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional research grants, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional research grants, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional research grants, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Loxo: Research Funding.
"Venetoclax is a BCL-2 inhibitor indicated: for treatment of adult patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL); in combination with azacitidine, or decitabine, or low-dose cytarabine to treat adults with newly-diagnosed acute myeloid leukemia (AML) in adults who are age 75 years or older, or who have comorbidities that preclude use of intensive induction chemotherapy. CLL/SLL: Dosing with venetoclax begins with a 5-week ramp-up."
Author notes
Asterisk with author names denotes non-ASH members.