Background:
Post-transplant lymphoproliferative disease (PTLD), is one of the major complications after organ transplantation. The aim of this study is to study the incidence, risk factors especially EBV status, histopathological types, management, and outcomes of PTLD in the pediatric groups.
Methods:
Following the PRISMA guideline, we performed a comprehensive literature search on PubMed, Cochrane Library, Embase, and clinicaltrials.gov from the past ten years on May 04, 2020. We used the MeSH terms of organ transplantation and lymphoproliferative disorders. The initial search revealed 1741 articles. We excluded all case reports, case series, pre-clinical trials, review articles, and meta-analysis. We found twenty-eight studies. We extracted the data for baseline characteristics, recipient & donor EBV status, immunosuppression used, type & stage of PTLD, organ system involved, duration between transplant and PTLD diagnosis, treatment, and mortality.
Results:
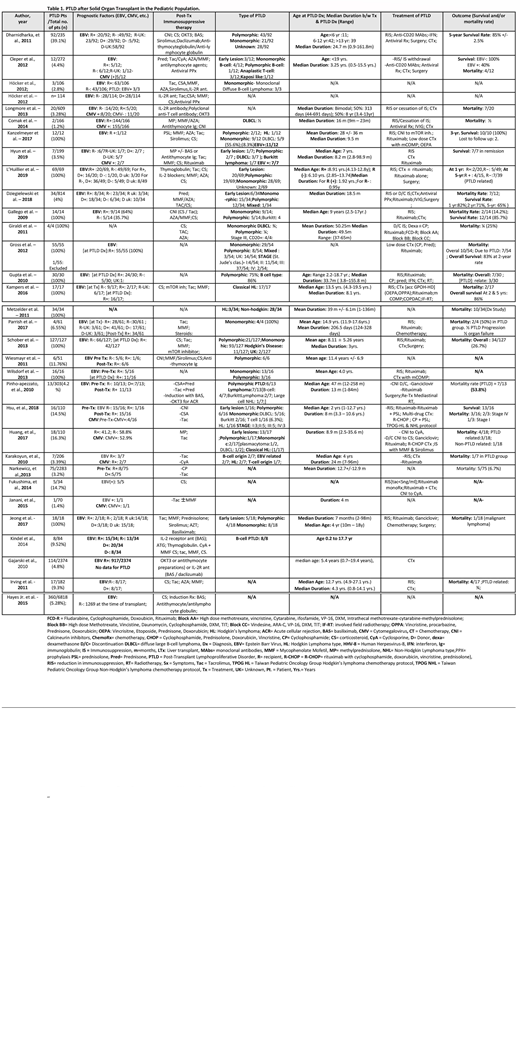
We included 32 studies with a total (n) of 15597 pediatric patients after a solid organ transplant. 1210 (7.75%) patients developed PTLD, out of which 609 (50.33%) were male participants, 453 (37.4%) female, and 148 (12.23%) the gender was unknown. (Table 1) Data from 19 studies showed that among PTLD patients, 115 recipients & 76 donors tested positive for Epstein-Barr Virus (EBV), 187 recipients were seronegative and the rest were unknown at the time of transplant. Immunosuppressive drugs used after organ transplantation included; cyclosporine, tacrolimus, sirolimus, azathioprine, mycophenolate, prednisone, mTOR inhibitors, induction therapy with OKT3, IL-2 inhibitors (basiliximab, daclizumab) and anti-thymocyte/anti-lymphocyte globulin. In two studies, antiviral prophylaxis with ganciclovir/valganciclovir was achieved. Data from nine studies showed the median age at PTLD diagnosis was 6.8 years. L'Huillier et al. stated that the median duration for EBV positive recipients to develop PTLD was 1.92 years (0.35-3.09 years), compared to EBV negative recipients, which was 0.95 years (2.48-2.92 years). Overall, the median duration from organ transplant to the diagnosis of PTLD among 15 studies was 29.1 months, ranging from 0.9 months to 186 months. The histopathology of PTLD was confirmed via biopsy specimen showing predominance in monomorphic type, i.e; 265/1210 (21.9%), followed by polymorphic type with 140/1210 (11.5%), an early lesion with 46/1210 (3.80%), Non-Hodgkin lymphoma 28/1210 (2.3%), Hodgkin lymphoma 33/1210 (2.72%) and mixed type (polymorphic & monomorphic) 4/1210 (0.33%). Histopathological data for 694 patients were not available. Treatment of PTLD was achieved with reduction or complete cessation of immunosuppressive therapy, anti-CD20 antibody (rituximab), antiviral treatment with ganciclovir/valganciclovir, lymphoma treatment with chemotherapeutic agents, radiotherapy, and surgical resection. Gajarski et al. concluded that patients who received induction therapy [OKT3, IL-2 antagonists (basiliximab, daclizumab) and anti-thymocyte globulin] had significantly less PTLD development as compared to those who did not receive induction therapy, i.e., (2.9% vs. 4.9%) among 2374 total patients. According to Hayes Jr. et al., recipient EBV seronegativity continued to be associated with lower mortality hazard (HR 0.715; 95 % CI 0.547, 0.935; p = 0.014). Data from 19 studies showed the mortality rate among 494 PTLD diagnosed patients was 22.7% (112) with an overall survival of 84.2% respectively from three studies. Data from five studies showed that direct PTLD related mortality accounted for 63% (17/27) of deaths in PTLD diagnosed patients.
Conclusion:
Our analysis shows that post-transplant EBV positive recipients who undergo multiorgan transplants are more likely to develop PTLD compared to EBV positive recipients with single organ transplantation. The median duration from organ transplants to the diagnosis of PTLD ranged from months to years, however, this duration was reported to be less in EBV negative recipients compared to EBV positive recipients with organ transplants. The most common histological type of PTLD among pediatric groups was monomorphic and the least common was Classic Hodgkin lymphoma. Rituximab use in the treatment of PTLD was associated with a decreased use of alkylating agents and as a result, the side effects associated with these agents were reduced.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.