Background. Refractory or relapsed mantle cell lymphoma (R/R MCL) is generally associated with poor outcomes; median overall survival (OS) is 4-5 years. First generation Bruton's tyrosine kinase inhibitor (BTKi) (Ibrutinib) and second generation BTKi (acalabrutinib and zanubrutinib) have led to significant improvements in efficacy and safety over conventional chemoimmunotherapy in treating R/R MCL. In the absence of direct head to head clinical trials compare between BTKi, indirect comparisons between the first and second BTKi generations show possible differences in safety and efficacy. We used existing evidence from phase I/II clinical trials for second BTKi generation to evaluate the cost-effectiveness of ibrutinib vs acalabrutinib vs zanubrutinib in treating patients with R/R MCL from the US payer perspective.
Methods. A Markov model with two health states (progression-free [PF] and progression or death) was specified. Kaplan-Meier (KM) curves of PF survival (PFS) from the phase III trial by Dreyling et al. (Lancet 2016) for ibrutinib, the phase II trial by Wang et al. (Lancet 2018) for acalabrutinib, and the phase I/II trial by Tam et al. (Blood 2019) for zanubrutinib were fitted to exponential distributions to extract transition probabilities between the two health states for each drug. Wholesale acquisition costs (WAC) were obtained from RedBook and costs of adverse events management were derived from the literature. The analysis was conducted over a lifetime horizon with health utility outcomes and costs discounted at 3.5% per year after the first year. The cost and PFS life years (LYs) and PFS quality-adjusted LYs (QALYs) for each treatment, the incremental PFS LYs and PFS QALYs gained with acalabrutinib or zanubrutinib over ibrutinib, and the incremental cost-effectiveness ratio (ICER) and cost-utility ratio (ICUR) were estimated in both base and probabilistic sensitivity analyses (PSA: 100,000 simulations).
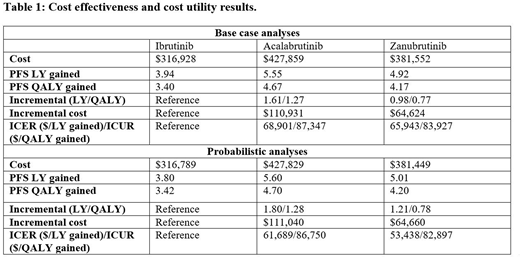
Results. As detailed in the table, acalabrutinib and zanubrutinib were associated with better clinical outcomes than ibrutinib, with incremental PFS LYs gained of 1.61 and 0.98, and incremental PFS QALYs of 1.27 and 0.77, respectively. The incremental costs when comparing acalabrutinib and zanubrutinib with ibrutinib were $110,931and $64,624, respectively. In probabilistic analyses, the ICERs ($61,689/LYg for acalabrutinib; $53,438/LYg for zanubrutinib) and ICURs ($86,750/QALYg for acalabrutinib; $82,897/QALYg for zanubrutinib) were lower than the US willingness to pay (WTP) threshold of $100,000 to $150,000 per QALY for cancer treatment. At WTP of $100,000, the cost-effectiveness acceptability curves showed the probabilities of acalabrutinib, zanubrutinib, and ibrutinib being cost-effective to be 50%, 34%, and 16%, respectively.
Conclusions. Acalabrutinib is more cost-effective compared with ibrutinib and zanubrutinib and improves health outcomes more in R/R MCL patients. This analysis using phase I/II trials should be validated as additional trial and real-world evidence about efficacy, safety, and associated health-related quality of life outcomes. Based on the current data, acalabrutinib offers the most cost-effective treatment option in R/R MCL.
McBride:Coherus BioSciences: Consultancy, Speakers Bureau; Merck: Speakers Bureau; Pfizer: Consultancy; Sandoz: Consultancy; MorphoSys: Consultancy; Bristol-Myers Squibb: Consultancy. Abraham:Janssen: Consultancy; Coherus BioSciences: Research Funding, Speakers Bureau; Celgene: Consultancy; Sandoz: Consultancy; MorphoSys: Consultancy; Mylan: Consultancy; Rockwell Medical: Consultancy; Terumo: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.