In this issue of Blood, Khan et al1 reported that high-dose chemotherapy and autologous hematopoietic cell transplantation (auto-HCT) are associated with decreased fecal microbiota diversity and that patients with a greater degree of microbiome damage (below-median fecal intestinal diversity) at neutrophil engraftment had worse progression-free survival than those with less microbiome damage.
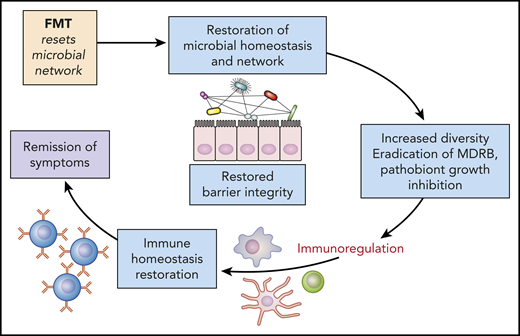
Microbiota modulation: potential mechanisms and restoration of homeostasis and gut barrier. Increasing the microbiota diversity can permit the regeneration of the gut barrier protective effect, while also curbing inflammation. FMT, fecal microbiota transfer; MDRB, multi-drug-resistant bacteria. Professional illustration by Patrick Lane, ScEYEnce Studios.
Microbiota modulation: potential mechanisms and restoration of homeostasis and gut barrier. Increasing the microbiota diversity can permit the regeneration of the gut barrier protective effect, while also curbing inflammation. FMT, fecal microbiota transfer; MDRB, multi-drug-resistant bacteria. Professional illustration by Patrick Lane, ScEYEnce Studios.
Over the last decade, with the development of metagenomic analysis, comprehensive analysis of the human gut microbiome has become possible and has shown the major role played by microbiota in health and disease.2 Therefore, perturbation of the gut microbiota, hereafter referred to as dysbiosis, has been associated with multiple diverse diseases, including infection, cancer, inflammatory and metabolic disease, neurological disease, and mental illness.2 In the field of hematologic malignancies, microbiota research has mainly focused on allogeneic HCT, where fecal microbiota dysbiosis has been associated with bloodstream infections, graft-versus-host disease, and shorter overall survival.3 Furthermore, a higher abundance of a bacterial group composed mostly of Eubacterium limosum has been associated with a decreased risk of relapse/progression of the hematologic malignancy after allo-HCT.4 Importantly, use of broad-spectrum antibiotics during allo-HCT is an important driver of gut microbiota dysbiosis and negatively impacts patient outcomes.5
The question is now whether a similar correlation between gut microbiota injury and patient outcome exists with other therapeutic strategies used in patients with hematologic malignancies. The question is particularly relevant for auto-HCT, given the exposure to high-dose chemotherapy, broad-spectrum antibiotics, mucositis, and impaired nutritional status, that may induce gut microbiota dysbiosis. Khan et al performed the largest and first multicenter study in 534 adult patients undergoing auto-HCT for myeloma or lymphoma. They found that, similar to allo-HCT, fecal microbiota diversity at baseline (ie, before auto-HCT) is decreased compared with healthy volunteers. Furthermore, patients experienced further microbiota diversity decrease during the neutropenic period with a nadir around engraftment. Greater fecal microbiota diversity at engraftment was then associated with a decreased risk of progression or death even after adjusting for disease type and disease status (hazard ratio [HR] 0.6; 95% confidence interval [CI], 0.42 to 0.83, P = .006). However, although greater fecal microbiota diversity at engraftment was also associated with an improved overall survival, the association was not statistically significant after adjusting for disease type and status.
This study included myeloma and different lymphoma subtypes, and we can question the impact of that important heterogeneity on the findings. In an attempt to overcome this heterogeneity, the authors analyzed a more homogenous group of 99 patients with myeloma who underwent auto-HCT within 12 months of induction chemotherapy. Higher fecal microbiota diversity at engraftment was again associated with a reduced risk of progression or death even after adjusting for cytogenetic risk (HR, 0.53; 95% CI, 0.29-0.96; P = .037). Nevertheless, only 99 patients were included in this post hoc analysis, and further studies are needed to confirm their findings.
Overall, Khan et al’s results confirmed that, as suggested, the microbiota is associated with patient outcome after auto-HCT, similarly to what was reported after allo-HCT. However, it remains to establish the directionality of this relationship, for instance, is a decrease in gut microbiota diversity responsible for the increased risk of progression or death or is a decreased gut microbiota diversity a surrogate marker of other parameters that impact patient outcome. Previous studies may suggest some answers. In a myeloma mouse model, gut microbiota manipulation drives interleukin-17–producing cells and eosinophils that synergize to accelerate myeloma progression.6 Furthermore, in a group of 34 myeloma patients receiving first-line treatment, a correlation between microbiota composition (higher relative abundance of Eubacterium hallii) and achievement of minimal residual disease negativity was reported,7 further supporting a role of microbiota in multiple myeloma progression. Nevertheless, there was no correlation between bacteria associated with myeloma progression in those studies, and the risk of death or progression in the current study by Khan et al.
Finally, this study raises the question of the role of therapeutic strategies to prevent or restore intestinal dysbiosis in those patients. Several approaches could be used and even combined, including antibiotic-sparing strategies, and administration of prebiotics (such as nondigestible carbohydrate), postbiotics (such as short-chain fatty acids), or probiotics. Regarding probiotics, it may be difficult to identify relevant selected strains to transfer, because, as presented here, bacteria identified in 1 study are often not confirmed in a subsequent study. In contrast, it has already been demonstrated that fecal microbiota transfer, in the setting of allo-HCT8 and graft-versus-host disease therapy,9 and after induction chemotherapy for acute myeloid leukemia, is safe and effective in restoring gut microbiota diversity.10 Increasing the microbiota diversity can help regeneration of the gut barrier protective effect and can curb inflammation (see figure). Therefore, fecal microbiota transfer may be considered an adjuvant therapy during auto-HCT aimed at restoring gut microbiota diversity from baseline until patients’ full recovery.
In summary, microbiota diversity is increasingly recognized as a key prognostic stratification factor in hematologic malignancies. An altered state of host-microbe crosstalk with autoaggravating signals from both sides may worsen the outcome of patients. Therefore, microbiota modulation should be considered as a target for personalized nutrition and therapy. The work by Khan et al paves the way for such interventions.
Conflict-of-interest disclosure: F.M. reports lecture honoraria from Therakos/Mallinckrodt, Biocodex, Janssen, Keocyt, Sanofi, Jazz Pharmaceuticals, and Astellas. M.M. reports grants and/or lecture honoraria from Janssen, Sanofi, MaaT Pharma, JAZZ Pharmaceuticals, Celgene, Amgen, BMS, Takeda, Pfizer, and Roche.