Abstract
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is a potentially fatal thrombotic microangiopathy caused by autoantibody-mediated severe deficiency of ADAMTS13. Standardized definitions of response, exacerbation, remission, and relapse were initially proposed in 2003 and modified by the International Working Group for TTP in 2017. These definitions, which have been widely used in clinical practice and research, are based primarily on the platelet count and are benchmarked against the timing of discontinuation of therapeutic plasma exchange (TPE). They do not incorporate ADAMTS13 activity or the temporizing effects on the platelet count of caplacizumab, a novel anti–von Willebrand factor (VWF) nanobody. In light of these limitations, the IWG aimed to develop revised consensus outcome definitions that incorporate ADAMTS13 activity and the effects of anti-VWF therapy, by using an estimate-talk-estimate approach. The updated definitions distinguish clinical remission and clinical relapse (defined primarily by platelet count) from ADAMTS13 remission and ADAMTS13 relapse (defined by ADAMTS13 activity). The revised definitions of exacerbation and remission are benchmarked against not only the timing of discontinuation of TPE but also that of anti-VWF therapy. Retrospective validation of the revised definitions is described, although they have yet to be prospectively validated. Clinical implications of the updated outcome definitions are also discussed and an example of their application to clinical practice is provided to highlight their clinical relevance.
Introduction
Immune thrombotic thrombocytopenic purpura (iTTP) is a thrombotic microangiopathy caused by the autoantibody-mediated absence or severe deficiency of ADAMTS13, a plasma metalloprotease necessary for cleavage of the ultralarge multimers of von Willebrand factor (VWF).1,2 In the presence of ADAMTS13, the newly synthesized and secreted ultralarge multimers of VWF are cleaved into smaller multimers that circulate in a folded form with their platelet-binding A1 domains hidden.2 In the absence of ADAMTS13, the ultralarge multimers of VWF persist in circulation. The shear forces of flowing blood cause the ultralarge VWF multimers to unfold, exposing their A1 domains so that they become hyperadhesive for platelets.3 The resulting platelet- and VWF-rich microthrombi obstruct the microcirculation, leading to thrombocytopenia with the mechanical hemolysis, microvascular thrombosis, and ischemic organ injury that are the hallmarks of iTTP.4
Clinical outcome criteria in iTTP were first proposed in 2003.5 At that time, the initial treatment was therapeutic plasma exchange (TPE)6 and corticosteroids.7 Rituximab treatment of iTTP had been reported,8 but had not yet been systematically investigated.9 The association of ADAMTS13 deficiency with TTP had only recently been described,10,11 and ADAMTS13 activity was not routinely measured in clinical practice. In that era, the platelet count was the principal parameter for estimating the response to treatment and the achievement of remission. Attaining a normal platelet count indicated the response of iTTP to treatment and a sustained normal platelet count for 30 days after discontinuation of TPE indicated a durable remission. Recurrences of iTTP within and beyond 30 days after cessation of TPE were described as exacerbation and relapse, respectively.
In 2017, we (the International Working Group [IWG] for TTP) published modified outcome definitions.1 These definitions describe the course and outcomes in TTP (Table 1) and have been widely used in clinical practice and in research studies.
However, several recent advances in the management of iTTP mandate updates to these definitions. First, ADAMTS13 activity measurement is now more widely available and increasingly used in clinical practice, not only for diagnosis, but also as a marker of risk for recurrence and response to therapy.12-14 Second, immunosuppressants (rituximab, in particular) have been demonstrated to reduce formation of anti-ADAMTS13 autoantibodies, thereby increasing ADAMTS13 activity levels and mitigating the risk of exacerbation and relapse.9,15-18 Third, caplacizumab, an anti-VWF nanobody that binds to the A1 domain of VWF, was recently approved in various jurisdictions for the initial treatment of iTTP in conjunction with TPE,19,20 and other anti-VWF therapies are in development.21,22 Finally, although TPE remains the standard initial treatment, recombinant ADAMTS13 infusion is under investigation for the management of congenital TTP23 and iTTP (registered at www.clinicaltrials.gov as #NCT03922308) and may complement or supplant TPE in the future.
In light of these dramatic changes in the therapeutic landscape, revised outcome definitions are needed that include not only conventional markers of disease activity, such as the platelet count, but also incorporate ADAMTS13 activity. Revised outcome definitions are also needed that account for the impact of anti-VWF therapy on the platelet count. Herein, we summarize the limitations of our previous definitions of response, exacerbation, remission, and relapse in the context of contemporary practice (Table 1) and we propose updated definitions (Table 2) that reflect current iTTP management. Using a case study, we also discuss the management implications of our revised definitions and highlight their clinical relevance (Figure 1).
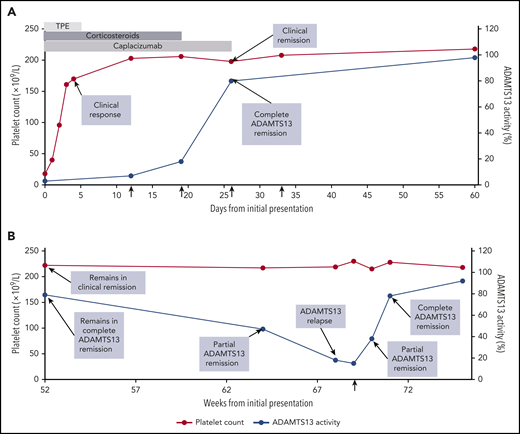
Clinical course of patient with iTTP. The clinical course of a 32-year-old woman with iTTP during the first 60 days (A) and at 1 year (B). She presented on day 0 with petechiae, abdominal pain, a platelet count of 18 × 109/L (normal range, 150 × 109/L to 400 × 109/L), a markedly elevated LDH, and schistocytes on the peripheral blood smear (A). ADAMTS13 activity was 3% (normal, ≥67%) with a detectable functional inhibitor, confirming the diagnosis of iTTP. TPE was initiated (light gray bar), along with corticosteroids (dark gray bar) and caplacizumab (medium gray bar). She improved rapidly with resolution of the abdominal pain, reduction in LDH level, and normalization of the platelet count on day 3. This improvement was sustained over the next several days, constituting a clinical response. TPE was discontinued on day 5, the platelet count remained stable, and she was discharged home on day 7 with prescriptions for caplacizumab and a prednisone taper. On day 12, despite a normal platelet count, she had persistent severe ADAMTS13 deficiency (7%). That day, rituximab was prescribed at 375 mg/m2 × 1 dose per week for 4 weeks (vertical arrows). By day 19, the ADAMTS13 level had risen to 18%, and by day 26, it had risen to 80%, indicating a complete ADAMTS13 remission and attainment of clinical remission. Based on the demonstration of ADAMTS13 recovery, caplacizumab was discontinued on day 26. The patient completed the planned 4-week course of rituximab. The patient continued to be observed with periodic clinic visits and blood work during remission. At the 52-week visit (B), she remained in clinical remission and complete ADAMTS13 remission with a normal platelet count and an ADAMTS13 level of 79%. Twelve weeks later (week 64), the platelet count remained normal, but the ADAMTS13 level had declined to 47%. She was reclassified as being in clinical remission and partial ADAMTS13 remission and instructed to return for blood work in 4 weeks. At 68 weeks, she remained in clinical remission, but the ADAMTS13 level had declined to 18%, which qualified as an ADAMTS13 relapse. A test for ADAMTS13 level, repeated several days later, showed 15%. Preemptive rituximab was recommended to reduce the risk of clinical relapse. That day, the patient was treated with a single dose of rituximab 375 mg/m2 (vertical arrow; B). Rituximab was successful in reinducing ADAMTS13 remission. One week later, the ADAMTS13 level had risen to 38% (partial ADAMTS13 remission) and by 2 weeks, it had risen to 78% (complete ADAMTS13 remission). Two years later, the patient remained in clinical remission and complete ADAMTS13 remission without the need for additional immunosuppression. This case was selected to highlight the updated outcome definitions (Table 2). It is not intended to endorse specific management strategies.
Clinical course of patient with iTTP. The clinical course of a 32-year-old woman with iTTP during the first 60 days (A) and at 1 year (B). She presented on day 0 with petechiae, abdominal pain, a platelet count of 18 × 109/L (normal range, 150 × 109/L to 400 × 109/L), a markedly elevated LDH, and schistocytes on the peripheral blood smear (A). ADAMTS13 activity was 3% (normal, ≥67%) with a detectable functional inhibitor, confirming the diagnosis of iTTP. TPE was initiated (light gray bar), along with corticosteroids (dark gray bar) and caplacizumab (medium gray bar). She improved rapidly with resolution of the abdominal pain, reduction in LDH level, and normalization of the platelet count on day 3. This improvement was sustained over the next several days, constituting a clinical response. TPE was discontinued on day 5, the platelet count remained stable, and she was discharged home on day 7 with prescriptions for caplacizumab and a prednisone taper. On day 12, despite a normal platelet count, she had persistent severe ADAMTS13 deficiency (7%). That day, rituximab was prescribed at 375 mg/m2 × 1 dose per week for 4 weeks (vertical arrows). By day 19, the ADAMTS13 level had risen to 18%, and by day 26, it had risen to 80%, indicating a complete ADAMTS13 remission and attainment of clinical remission. Based on the demonstration of ADAMTS13 recovery, caplacizumab was discontinued on day 26. The patient completed the planned 4-week course of rituximab. The patient continued to be observed with periodic clinic visits and blood work during remission. At the 52-week visit (B), she remained in clinical remission and complete ADAMTS13 remission with a normal platelet count and an ADAMTS13 level of 79%. Twelve weeks later (week 64), the platelet count remained normal, but the ADAMTS13 level had declined to 47%. She was reclassified as being in clinical remission and partial ADAMTS13 remission and instructed to return for blood work in 4 weeks. At 68 weeks, she remained in clinical remission, but the ADAMTS13 level had declined to 18%, which qualified as an ADAMTS13 relapse. A test for ADAMTS13 level, repeated several days later, showed 15%. Preemptive rituximab was recommended to reduce the risk of clinical relapse. That day, the patient was treated with a single dose of rituximab 375 mg/m2 (vertical arrow; B). Rituximab was successful in reinducing ADAMTS13 remission. One week later, the ADAMTS13 level had risen to 38% (partial ADAMTS13 remission) and by 2 weeks, it had risen to 78% (complete ADAMTS13 remission). Two years later, the patient remained in clinical remission and complete ADAMTS13 remission without the need for additional immunosuppression. This case was selected to highlight the updated outcome definitions (Table 2). It is not intended to endorse specific management strategies.
Methods
This document was developed by the IWG on TTP as an update to its previously proposed outcome definitions.1 We used an estimate-talk-estimate approach analogous to what is used in a modified Delphi process.24 Specifically, draft outcome definitions with open-ended questions were distributed to IWG members for their individual feedback before an in-person meeting. Members of the IWG subsequently met at the American Society of Hematology annual meeting (December 2019, Orlando, FL) where individual, anonymized feedback was presented to the panel to encourage consensus. After the meeting, revised definitions were sent to the panel for final approval.
The work of the group was supported by a literature search of the National Library of Medicine’s PubMed database and supplemented by review of the individual authors’ files. The revised outcome definitions in this report represent the consensus opinions of the authors. The work of the IWG was not funded.
Definitions
Clinical response
In the 2017 IWG report, clinical response was defined as a sustained platelet count ≥150 × 109/L and lactate dehydrogenase (LDH) <1.5 times the upper limit of normal (ULN; Table 1).1 Our revised definition of clinical response (Table 2) maintains the platelet count and LDH criteria and includes the additional criterion that there must be no clinical evidence of new or progressive ischemic organ injury (eg, cardiac or neurologic symptoms). In general, TPE may be discontinued soon after a clinical response is achieved.
We decided not to incorporate measurement of ADAMTS13 activity in our definition of response because the goal of TPE is to induce a clinical response. TPE is not expected to induce a rapid and durable recovery in ADAMTS13 activity.25 Rather, recovery of ADAMTS13 signals a remission and is discussed later in “Remission.”
Some patients do not achieve a clinical response after 5 TPE sessions or experience an initial improvement followed by a decrease in platelet count caused by iTTP while receiving TPE. According to the 2017 IWG criteria, these patients have refractory TTP.1 Use of anti-VWF therapy and immunomodulatory agents (eg, corticosteroids and rituximab) hold promise for significantly reducing the incidence of refractory disease.20
Clinical exacerbation
TPE is highly effective at improving the platelet count and reducing thrombotic complications and death in patients with iTTP.6 However, it does not address the underlying formation of anti-ADAMTS13 autoantibodies. Indeed, patients who respond to initial treatment with TPE may experience a sudden decline in their platelet count within 30 days (and often within just a few days) after TPE is discontinued, even when immunosuppressive treatments such as corticosteroids and rituximab have been initiated. This phenomenon, termed exacerbation in the 2017 IWG criteria (Table 1),1 is related to the effects of persistent antibody-mediated severe ADAMTS13 deficiency.
Similar to TPE, anti-VWF therapy is temporizing. It raises the platelet count and reduces thrombotic complications, but it does not correct the underlying autoimmune dysfunction responsible for severe ADAMTS13 deficiency, nor does it protect against iTTP recurrence after it is discontinued if severe ADAMTS13 deficiency persists.19,20 This was clearly demonstrated in the phase 2 TITAN trial.19 In that trial, 75 adults with iTTP were randomized to caplacizumab 10 mg daily or placebo during and for 30 days after discontinuation of TPE. Eleven patients (28%) in the placebo group experienced an exacerbation, whereas exacerbation occurred in only 3 patients (8%) in the caplacizumab arm. However, in the month after discontinuation of caplacizumab, a “catch-up” effect was observed.12 No patients in the placebo group experienced recurrent thrombocytopenia compared with 8 patients (22%) in the caplacizumab group, 7 of whom had persistent severe ADAMTS13 deficiency. In accordance with the 2017 IWG outcome definitions (Table 1),1 the episodes of recurrent iTTP in the 8 patients were classified as relapses rather than exacerbations because they arose ≥30 days after cessation of TPE, even though they occurred within 30 days of discontinuing caplacizumab.
We propose a revised definition of exacerbation that accounts for the temporizing effects of both TPE and anti-VWF therapy on platelet count (Table 2). After an initial clinical response and before a clinical remission is attained, a clinical exacerbation occurs when the platelet count decreases to <150 × 109/L (with other causes of thrombocytopenia excluded), with or without clinical evidence of new or progressive ischemic organ injury, within 30 days of stopping TPE or anti-VWF therapy. Based on our revised definition, the 8 episodes of recurrent iTTP that occurred in the TITAN trial more than 30 days after discontinuation of TPE would be reclassified as clinical exacerbations because they occurred within 30 days after discontinuation of caplacizumab and before attainment of clinical remission. Using our revised definition, the rate of clinical exacerbation was similar to that in the TITAN trial between the caplacizumab and placebo groups (31% vs 28%), just as one would predict for a temporizing therapy such as caplacizumab.
In patients with iTTP who have achieved a clinical response, the temporizing effects of anti-VWF therapy can be exploited to “buy time,” allowing immunosuppressive therapy (eg, rituximab) to suppress anti-ADAMTS13 autoantibody production so that ADAMTS13 recovery can occur. The value of this strategy was illustrated in the phase 3 HERCULES trial.20 The design of the HERCULES trial was similar to that of the phase 2 TITAN trial,19 with a key difference: investigators in the HERCULES trial were encouraged to extend the study drug for up to 28 additional days in patients with persistent, severe ADAMTS13 deficiency, while optimizing immunosuppressive therapy. Although the incidence of iTTP recurrence was similar between the caplacizumab and placebo groups in the phase 2 TITAN trial, the incidence of iTTP recurrence in the HERCULES trial was significantly reduced in the caplacizumab arm compared with the placebo (12% vs 38%; P < .001) during the overall trial (which included a 28-day follow-up period after completion of treatment with caplacizumab or placebo).12,19,20
As per the HERCULES protocol, we and others advocate measuring ADAMTS13 levels at presentation and weekly in patients receiving caplacizumab and discontinuing it once ADAMTS13 remission has been achieved, rather than stopping after an arbitrary period (eg, 30 days after discontinuation of TPE as in the TITAN trial19 ).12,13,26,27 When anti-VWF therapy is used in this fashion, clinical exacerbations are significantly reduced. Nevertheless, clinical exacerbations may still occur in cases where anti-VWF therapy is not used or is discontinued before ADAMTS13 recovery.20 An ADAMTS13 threshold above which anti-VWF therapy can be safely discontinued remains to be defined, although a level that is increasing and is >20% to 30% for at least 2 consecutive weeks has been suggested.12,13 In the recently reported German postmarketing experience with caplacizumab, there were no TTP recurrences when the drug was discontinued in patients with ADAMTS13 activity >10%.27
Remission
As mentioned, patients with iTTP who have an initial clinical response to TPE may experience a clinical exacerbation shortly after TPE is discontinued because of persistent severe ADAMTS13 deficiency. In recognition of this tendency, the 2017 IWG outcome criteria distinguished clinical remission (platelet count ≥150 × 109/L and LDH <1.5 times ULN for ≥30 days after cessation of TPE) from clinical response (Table 1).1 The previous definition of remission was important for differentiating transient from more durable responses, but was limited in 2 respects related to current practice. First, it used platelet count as the primary marker of remission and did not incorporate the concept of ADAMTS13 recovery. Second, it accounted for the temporizing effects of TPE on the platelet count, but did not account for the temporizing effect of anti-VWF therapy when it was discontinued before ADAMTS13 recovery occurred (Table 1).
To overcome these limitations, we propose definitions that distinguish clinical remission from ADAMTS13 remission and that are benchmarked against the timing of discontinuation of both TPE and anti-VWF therapy (Table 2). We define clinical remission as a sustained clinical response with either (1) no TPE and no anti-VWF therapy in the past 30 days or (2) attainment of ADAMTS13 remission (partial or complete), whichever occurs first. We define partial ADAMTS13 remission as ADAMTS13 activity ≥20% but less than the lower limit of normal (LLN) and complete ADAMTS13 remission as ADAMTS13 activity ≥LLN. Because there is biological variability in ADAMTS13 activity as well as variability in its laboratory measurement in patients with iTTP,28 it may be advisable to repeat measurement of ADAMTS13 activity to confirm an ADAMTS13 remission, relying more on the trend than a specific value.
By separating ADAMTS13 remission from clinical remission, our proposed definitions acknowledge that some patients may achieve a clinical remission without an ADAMTS13 remission. Those patients are at increased risk of clinical relapse.14-16,29 Immunosuppression (eg, rituximab) may be administered with the goal of inducing an ADAMTS13 remission, thereby reducing the risk of clinical relapse.9,15-18,30 On the other hand, our definitions reflect that ADAMTS13 remission is always accompanied by clinical remission. In patients receiving anti-VWF therapy, the drug can generally be stopped safely after, but not before, attainment of an ADAMTS13 remission. A potential exception is the 10% to 20% of patients with iTTP who do not achieve an ADAMTS13 remission, even after intensive immunosuppression.15,27 Although every effort should be made to optimize immunosuppression to induce an ADAMTS13 remission in such patients, it may be impractical to continue anti-VWF therapy indefinitely and the drug may need to be discontinued without attainment of ADAMTS13 remission.
We selected ADAMTS13 activity of 20% as a minimum threshold for partial ADAMTS13 remission based on limited evidence that an ADAMTS13 level at or above this threshold is protective against relapse.15-18,27 We acknowledge that the minimum ADAMTS13 level above which patients are protected from relapse is uncertain and requires further study. We also acknowledge that, whereas an ADAMTS13 level of ≥20% may be sufficient to prevent iTTP relapse, it may not be sufficient to prevent other adverse clinical outcomes. For example, emerging evidence suggests that, among patients with a history of iTTP who are in clinical remission, those with a partial ADAMTS13 remission are at greater risk of ischemic stroke than those with a complete ADAMTS13 remission.31 Elucidation is needed of biomarkers that are more predictive than ADAMTS13 activity, not only of iTTP relapse, but also of clinical manifestations that may occur in the absence of overt TTP. A promising candidate is ADAMTS13 conformation, but further investigation is warranted.32
Relapse
The 2017 IWG criteria define relapse as a decline in platelet count to <150 × 109/L after attainment of clinical remission (Table 1).1 As with the previous definition of remission, a limitation of this definition is that it does not distinguish clinical relapse from ADAMTS13 relapse. In our updated criteria, we propose separate definitions for clinical relapse and ADAMTS13 relapse (Table 2). We define clinical relapse as a decrease in platelet count to <150 × 109/L (with other causes of thrombocytopenia ruled out), with or without clinical evidence of new ischemic organ injury, after attainment of a clinical remission. A clinical relapse should always be confirmed by documentation of severe ADAMTS13 deficiency (ADAMTS13 activity < 10%). We define ADAMTS13 relapse as a decrease in ADAMTS13 activity to <20% after an ADAMTS13 remission (complete or partial). Because there is biological variability in ADAMTS13 activity, as well as variability in its laboratory measurement, it is advisable to repeat the ADAMTS13 level to confirm an ADAMTS13 relapse.
We selected ADAMTS13 activity <20% as the threshold for defining ADAMTS13 relapse based on limited evidence that an ADAMTS13 level at or above this cutoff is protective against clinical relapse.15-18,27 As discussed above, we acknowledge that the minimum ADAMTS13 level above which patients are protected from relapse is uncertain and requires further study.
By segregating clinical relapse from ADAMTS13 relapse, our proposed definitions acknowledge that an ADAMTS13 relapse can occur without a clinical relapse. In a patient in ADAMTS13 remission, ADAMTS13 activity may decline over time to <20%, resulting in an ADAMTS13 relapse, which is a well-established predictor of clinical relapse.14-16,28 Immunosuppression (eg, preemptive rituximab) may be used in such situations to reinduce an ADAMTS13 remission, thereby reducing the risk of clinical relapse (Table 2).9,15-18,30
Conclusion
The therapeutic landscape in iTTP has changed dramatically since the previous outcome criteria were proposed (Table 1).12,13,30 Although TPE and corticosteroids remain cornerstones of treatment, rituximab is now widely used for management of acute iTTP episodes and as a preemptive strategy for prevention of clinical relapse in patients who have had an ADAMTS13 relapse, but remain in clinical remission.15-18 Caplacizumab, the most recent addition to the iTTP armamentarium, is now approved for initial treatment together with TPE and other anti-VWF agents are in development.21,22 When used in conjunction with immunosuppression until attainment of ADAMTS13 remission, anti-VWF therapy has been shown to reduce the risk of clinical exacerbation.19,20 Recombinant ADAMTS13 is in development (registered at www.clinicaltrials.gov as #NCT03922308) as an additional therapeutic option for acute episodes.23 Measurement of ADAMTS13 activity is now widely used, not only for diagnosis, but also to guide management after a clinical response and during clinical remission.12,13,27 The inclusion of ADAMTS13 activity in our updated outcome definitions underscores the necessity of providing ADAMTS13 assay results in a clinically relevant time frame (ideally, within 72 hours33 ) to inform real-time treatment of patients with iTTP.
Our updated outcome definitions (Table 2) account for and incorporate these recent changes in patient care. An example of how they may be applied to a patient is provided in Figure 1. By distinguishing between clinical and ADAMTS13 remission and relapse, our definitions acknowledge the primacy of ADAMTS13 activity as a predictor of clinical exacerbation and clinical relapse and as a means of guiding therapeutic decisions. By benchmarking our definitions of clinical exacerbation and clinical remission against the timing of suspension of not only TPE but also of anti-VWF therapy, our criteria account for the temporizing effects of both treatments on the platelet count, which contrast with the more durable effects of immunosuppression.
An important limitation of our revised outcome definitions (Table 2) is that they have not been prospectively validated. We encourage their validation in future studies and in studies that have already been completed, much as we evaluated our revised definition of clinical exacerbation in the TITAN trial (see“ Clinical exacerbation”). Indeed, a strength of our outcome definitions is that they are based on simple parameters (ie, platelet count, LDH level, ADAMTS13 activity, timing of discontinuation of TPE, and timing of discontinuation of anti-VWF therapy), which are typically collected as part of routine care for patients with iTTP. Availability of these data allows for straightforward application of our revised definitions, not only in prospective fashion, to clinical practice and future studies, but also in retrospective fashion to studies that have already been completed, thereby facilitating comparison between past and future investigations. A second strength of our definitions is that they may be applied irrespective of whether a patient received anti-VWF therapy or other drug therapies. This is an important advantage because caplacizumab is not universally available and because concerns about its cost-effectiveness may limit its use, even in jurisdictions where it has been approved.34
We hope that these updated definitions will be useful in clinical practice and will serve as a template for standardized definitions in iTTP research. Undoubtedly, further revisions will be necessary in the future to keep pace with the inexorable advances in our understanding and management of iTTP.
The online version of this article contains a data supplement.
Authorship
Contribution: All authors provided input on the consensus definitions; A.C. searched the literature and wrote the paper; and all remaining authors critically revised the paper.
Conflict-of-interest-disclosure: A.C. has served as a consultant for Synergy, and his institution has received research support on his behalf from Alexion, Bayer, Novo Nordisk, Pfizer, Spark, and Takeda. S.R.C. has served as a consultant for Sanofi-Genzyme. P.C. has served as a consultant for Sanofi, Alexion, Takeda, and Roche. J.d.l.R. has served as a consultant and provided expert testimony for Sanofi. K.D.F. has served as a consultant for Alexion, Instrumentation Laboratories, Sanofi, and Takeda. P.N.K. has served on an advisory panel and has received speaker fees and travel grants from Ablynx/Sanofi, Alexion, Baxalta/Shire/Takeda, CSL-Behring, Nov-Nordisk, and Roche. J.A.K.H. has served as a consultant for Sanofi and Takeda and has received research support from Shire. B.L has served as a consultant for Sanofi/Ablynx; has served as chairman of the Data Safety Monitoring Board of studies of rhADAMTS13 for Takeda; and has received lecture fees/travel support from Baxter, Ablynx, Alexion, Siemens, Bayer, Roche, and Sanofi. M.M. is an inventor of an enzyme-linked immunosorbent assay (ELISA) for assessing ADAMTS13 activity and has received consultant/advisor fees from Chugai Pharmaceutical, Takeda, and Sanofi. K.P. has received honoraria from Ablynx/Sanofi, Shire/Takeda, and Alexion and has participated in clinical trials of Ablynx/Sanofi. F.P. has received honoraria for participating as a speaker at educational and satellite symposia organized by Roche, Sanofi, SOBI, Spark, and Takeda and has served on advisory boards for Roche, Sanofi, and SOBI. M.R.T. has received speaker’s fees and honoraria from Sanofi and Bayer. A.V. has served as a consultant for Sanofi, Takeda, and Roche. J.-P.W. has received speaker’s fees and honoraria from Alexion, Novartis, and Sanofi. M.S. has received speaker’s fees and honoraria from Alexion, Sanofi, Novartis, and Takeda and has received research funding from Takeda. The remaining authors declare no competing financial interests.
The members of the International Working Group for Thrombotic Thrombocytopenic Purpura are shown in the supplemental Appendix (available on the Blood Web site).
Correspondence: Adam Cuker, Hospital of the University of Pennsylvania, 3400 Spruce St, Philadelphia, PA 19104; e-mail: adam.cuker@pennmedicine.upenn.edu.