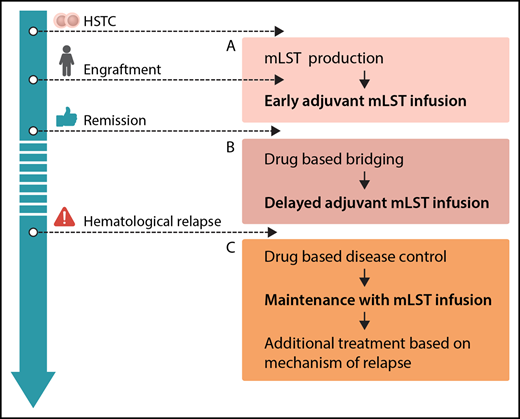
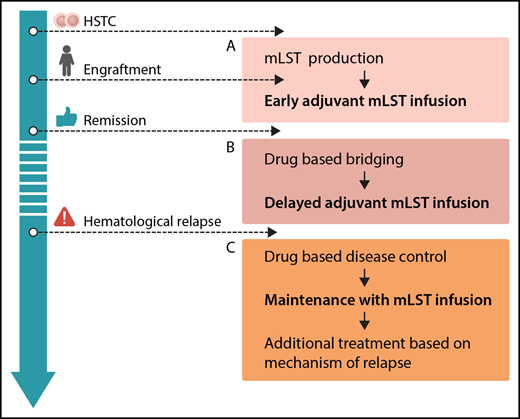
Options for integration of mLSTs into multimodal concepts for prevention and treatment of leukemia relapse after hematopoietic stem cell transplantation (HSCT): production before HSCT or during engraftment, allowing for early adjuvant mLST infusion (A), drug-based bridging after engraftment until delayed adjuvant mLST infusion (B), and combining drug-based disease control of overt hematological relapse, followed by maintenance with mLST infusion and additional treatment based on the mechanism of relapse (C). Professional illustration by Somersault18:24.
Options for integration of mLSTs into multimodal concepts for prevention and treatment of leukemia relapse after hematopoietic stem cell transplantation (HSCT): production before HSCT or during engraftment, allowing for early adjuvant mLST infusion (A), drug-based bridging after engraftment until delayed adjuvant mLST infusion (B), and combining drug-based disease control of overt hematological relapse, followed by maintenance with mLST infusion and additional treatment based on the mechanism of relapse (C). Professional illustration by Somersault18:24.
In this issue of Blood, Lulla et al report on successful generation and safe clinical application of multiple leukemia antigen–specific T cells (mLSTs) derived from healthy stem cell donors. The mLSTs were given to recipients of hematopoietic stem cell transplantation (HSCT) with high-risk myeloid malignancies.1 Safety was demonstrated by a low incidence of graft-versus-host disease (GVHD) after transfusion of the mLSTs. Evidence for clinical efficacy was shown by long-term remission in patients receiving adjuvant mLSTs, by exploring immune escape mechanisms in patients who did relapse after mLST infusion, and by clinical responses in patients with overt relapse.
Alloreactive T-cell responses are the basis for the graft-versus-leukemia (GVL) effect of allogeneic HSCT. This response is triggered by the recognition of peptides arising from differences in genomic polymorphisms between patient and donor. The antimalignant efficacy can be augmented by donor lymphocyte infusion (DLI), in both prevention and treatment of relapse.2 Minor histocompatibility antigens (MiHAs) restricted to the hematopoietic compartment, or nonmutated polymorphic self-antigens, preferentially expressed or overexpressed in malignant cells (tumor-associated antigens, TAAs) represent potential targets. However, the clinical utility of unselected DLI is limited by 2 factors: (i) DLI has the risk of severe GVHD, if nonhematopoietic tissues of the recipient are recognized and targeted; (ii) the number and avidity of T cells that recognize either hematopoietic tissue-restricted MiHAs or TAA are low in the peripheral blood of healthy individuals.3 This is a significant problem, as the GVL reaction depends on magnitude and diversity of the allogeneic T-cell response.4 A polyclonal T-cell response against different antigens is thought to be mandatory for the effective and persistent elimination of malignant cells.5 Hence, approaches with a lower risk of unwanted alloreactivity causing GVHD and a more effective and diverse GVL reaction are warranted.
Prior strategies to selectively target polymorphic antigens by donor T cells included gene transfer of T-cell receptors specific for defined peptide/HLA complexes with exclusive expression on either recipient hematopoietic cells (eg, the MiHA HA-1/HLA A*2)5 or malignant cells (eg, WT-1/HLA*24:02).6 Alternatively, TAA-specific T cells were purified using selection methods like streptamer technology. For example, WT-1–specific cytotoxic T cells could be selected for specific DLI.7 However, the focus on a single antigen and the restriction to 1 defined HLA type have limited the clinical success of these approaches.
Lulla et al have successfully addressed some of these obstacles by selection and expansion of naturally occurring donor T cells that were reactive to a battery of TAA, commonly expressed on malignant myeloid cells. Hence, this strategy can be used irrespective of the HLA type of the patient, and an immune escape of antigen-negative variants becomes less likely. High numbers of mLSTs were generated from all donors, enabling transfusion in a prospective clinical phase 1 trial to patients in remission but at high risk of relapse (ie, adjuvant use) and to patients with overt relapse. A dose-escalating regimen was used to define dose-limiting toxicities. All cellular products demonstrated <10% specific lysis of nonmalignant donor cells in vitro, which was used as a functional marker for the risk of causing GVHD. With development of mild GVHD in only 4/25 enrollees, this strategy proved to be extremely effective. No other safety issues were observed.
Although leukemia relapse is still the major cause of treatment failure after SCT, our knowledge about the biology of relapse has increased enormously.8 Relapse mechanisms include clonal evolution of the leukemia, loss of polymorphic target antigens, and evasion of malignant cells into sanctuary sites with weaker immunosurveillance, such as central nervous system or testes. Besides, the loss of the mismatched HLA genes after haploidentical SCT and the downregulation of HLA class II molecules in the HLA-matched setting can impair leukemia cell recognition. Further mechanisms include the expression of inhibitory immune checkpoint molecules and the induction of an anti-inflammatory cytokine milieu by the leukemic cells. Understanding of these mechanisms will be essential in the future to develop individual treatment strategies. In the present paper, Lulla et al used the monitoring of several relapse mechanisms to demonstrate the increased immune pressure caused by the transfused mLSTs. Indeed, leukemic blasts from all 5 patients who relapsed despite adjuvant mLSTs showed one or more of the described immune escape mechanisms. Conversely, this was not the case among controls with posttransplant relapse who had not received mLSTs.
From the clinician’s perspective, mLSTs represent a promising platform for consolidation strategies posttransplant. In general, future efforts should be put on standardization and simplification of the production protocol. Generation of mLSTs, as with many other approaches using specific T cells, is still complex. The ability to expand this technology to different centers has to be proven. Several scenarios for clinical application can be envisioned (see figure): adjuvant infusion early after SCT (see figure panel A) is attractive, because leukemia burden is low, and effective interventions would avoid early relapse, which is associated with a dismal prognosis. However, in the present study, this was prevented by a long production time with a median of 34 days of culturing. Therefore, strategies to start production of mLSTs before HCST or in parallel to the engraftment period, reduction of production times, or early posttransplant treatment with specific drugs like tyrosine kinase or histone deacetylase inhibitors prior to delayed adjuvant mLST application (see figure panel B) could be considered. With regard to treatment of overt hematological relapse (see figure panel C), the results of this trial underscore the limited efficacy of allogeneic T cells when given to patients with active leukemia, although clinical effects of mLSTs were observed in 2/8 patients. This has been shown repeatedly, regardless of the used strategy.9 Therefore, mLSTs will most likely not be a suitable monotherapy in patients with overt relapse either, but might be successfully used for consolidation after disease control by chemotherapy or novel drugs. As shown in the paper, immune escape strategies of malignant cells are also active in the context of mLSTs. Hence, combinations with drugs specifically thought to overcome these escape strategies (such as checkpoint inhibitors) might be a future option.
In summary, after establishing improved production strategies and integration into multimodal therapy, the described approach might become a safe and effective instrument for prevention and treatment of posttransplant relapse.
Conflict-of-interest disclosure: The author declares no competing financial interests.