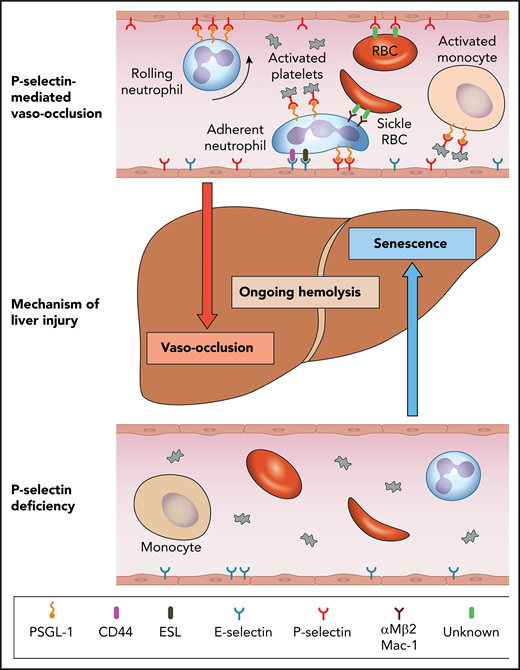
Model for mechanisms of liver injury in SCD as it relates to P-selectin deficiency. The top panel shows the role of P-selectin in mediating multicellular adhesive interactions leading to liver injury by vaso-occlusion. The bottom panel shows that the absence of P-selectin results in amelioration of these adhesive effects and lack of leukocyte recruitment to the endothelium, which lead to liver injury by senescence. Hemolysis is not improved by P-selectin deficiency. ESL, E-selectin ligand; PSGL-1, P-selectin glycoprotein ligand-1; RBC, red blood cell. Professional illustration by Patrick Lane, ScEYEnce Studios.
Model for mechanisms of liver injury in SCD as it relates to P-selectin deficiency. The top panel shows the role of P-selectin in mediating multicellular adhesive interactions leading to liver injury by vaso-occlusion. The bottom panel shows that the absence of P-selectin results in amelioration of these adhesive effects and lack of leukocyte recruitment to the endothelium, which lead to liver injury by senescence. Hemolysis is not improved by P-selectin deficiency. ESL, E-selectin ligand; PSGL-1, P-selectin glycoprotein ligand-1; RBC, red blood cell. Professional illustration by Patrick Lane, ScEYEnce Studios.
In this issue of Blood, Vats et al examine the effect of the absence of genetic P-selectin on hepatobiliary injury in mice with P-selectin–deficient sickle cell disease (SCD).1 As would be expected, there is the welcome attenuation of liver ischemia resulting from the inhibition of multicellular adhesive interactions. This improvement in vaso-occlusion is unfortunately offset by an increase in liver senescence, resulting in a lack of protection from the progressive liver damage that develops in SCD mice.
Although the seminal event in SCD is the polymerization of abnormal hemoglobin, the downstream pathophysiology is multifaceted and results from heterotypic multicellular adhesive interactions, ischemia reperfusion injury, hemolysis, and heightened inflammation.2 The considerable burden of this chronic disease manifests as pain, hemolytic anemia, organ injury, poor quality of life, and early mortality.3 Increased understanding of the complex pathophysiology has resulted in a plethora of novel agents in various stages of development. One such therapeutic target is P-selectin, a cellular adhesion molecule that is expressed on the endothelium and activated platelets and plays an important role in vaso-occlusion in SCD (see figure). P-selectin mediates initial capture, rolling, and recruitment of leukocytes on the activated vascular endothelium and the interactions between activated platelets and leukocytes. The interaction with platelets activates neutrophils and monocytes, which contribute to inflammation.4 P-selectin–deficient mice exhibit elevated numbers of circulating neutrophils, dramatic reduction in leukocyte rolling, and delayed extravasation of neutrophils to sites of inflammation.5
Vats et al use quantitative live intravital microscopy to evaluate the liver in P-selectin–deficient SCD mice. Notably, P-selectin deficiency does not result in the prevention of hepatobiliary injury despite improvement in liver sinusoidal vaso-occlusion, ischemia, and concomitant improvement in transaminases. The authors attribute this to the observed impaired migration of leukocytes into the liver tissue, increased cellular senescence, reduced epithelial proliferation, and decreased iron clearance in the liver. There is also evidence for increased fibrosis accompanied by an increase in direct bilirubin levels. As these mice age, the iron accumulation and liver injury progresses. There is a no improvement in hemolysis, based on levels of heme oxygenase-1 in the liver or on hemoglobin or reticulocyte counts. The differential effect of P-selectin in acute ischemia and liver damage is consistent with the reported uncoupling between the protection of acute vaso-occlusion and liver damage in SCD mice lacking the β2-integrin Mac-1.6
Vats et al propose that at the heart of these changes is the inability of leukocytes to migrate into tissues without the essential first step of being recruited to the endothelium via P-selectin–mediated adhesion. The authors posit the role of leukocytes in tissue homeostasis, injury resolution, removal of dead cells, and repair as being key to the observed abnormalities.7,8 Previous preclinical studies by Zhang et al9 have shown that neutrophil proinflammatory activity correlates positively with their ageing while in circulation. These aged neutrophils, under inflammatory conditions, represent an overly active subset that promotes tissue injury and is dramatically expanded in P-selectin–deficient mice. Zhang et al also demonstrated that reducing the number of circulating aged neutrophils (by depletion of the gut microbiota) significantly and dramatically improves inflammation-related organ damage in mouse models of SCD.9 Vats et al show a significant elevation of neutrophil (threefold) and monocyte (twofold) counts in the P-selectin–deficient SCD mice. Their contribution to liver injury is likely complex. Are some of the leukocytes damage promoting (eg, aged neutrophils) and others protective but not able to localize appropriately?
The study by Vats et al has particular clinical relevance in that on 15 November 2019, the US Food and Drug Administration approved crizanlizumab, a humanized monoclonal antibody against P-selectin, to reduce the frequency of vaso-occlusive crises in patients with SCD. This followed the completion of a successful phase 2 multicenter study, the SUSTAIN Trial.10 A total of 198 patients were randomly assigned to receive 14 doses of study drug (high and low doses) vs placebo administered intravenously over 52 weeks. A 45% reduction in the frequency of SCD-related pain crises was achieved with high-dose (5 mg/kg) crizanlizumab treatment vs placebo (median, 2.98 vs 1.63 crises per year; P = .01). This was regardless of concomitant hydroxyurea usage or frequent previous pain crises. There were no significant differences in adverse events reported. This was a big step forward in the therapeutic armamentarium of SCD.
The study by Vats et al provides critical insights into the potential negative consequences of P-selectin inhibition that deserve careful consideration. There are, however, noteworthy differences between the findings in the SUSTAIN trial and the murine studies reported by Vats et al. There are no reports of treatment-related abnormal liver function tests or increases in leukocyte counts in patients in the study. A case report (referenced by Vats et al) describes a transient elevation of leukocyte counts in a patient after treatment with crizanlizumab, but the finding was also associated with a severe infusion reaction that triggered a pain crisis. What could be contributing to these differences? P-selectin antibody–mediated inhibition in SCD patients likely differs from a genetic deficiency of P-selectin in mice. These differences could include degree of inhibition, duration of the effect, timing of onset of the inhibition, and species differences. Additional preclinical studies can interrogate some of these questions, but longer-term follow-up in patients is vital to further evaluate these concerns. It will be essential to monitor real-world evidence or data from well-designed natural history cohorts, as post-marketing data can be fraught with reporting bias.
Laboratory markers of hemolysis were systematically evaluated in the SUSTAIN trial, and there were no differences in the treatment cohort, similar to what is reported by Vats et al. In the current treatment era, it would be prudent for patients to continue hydroxyurea therapy while receiving crizanlizumab. In addition to other mechanisms, the targeted myelosuppression and reduction in hemolysis resulting from hydroxyurea could provide some protection against ongoing organ damage. Similarly, as novel agents become available, new therapeutic combinations may better target the complex pathophysiology of SCD. Finally, the study by Vats et al highlights the potential for novel therapeutic approaches to perturb intricate physiologic balances.
Conflict-of-interest disclosure: D.M. served as a consultant for Pfizer, Novartis, Forma Therapeutics, Global Blood Therapeutics, and bluebird bio.