Abstract
A majority of children and young adults with acute lymphoblastic leukemia (ALL) are cured with contemporary multiagent chemotherapy regimens. The high rate of survival is largely the result of 70 years of randomized clinical trials performed by international cooperative groups. Contemporary ALL therapy usually consists of cycles of multiagent chemotherapy administered over 2 to 3 years that includes central nervous system (CNS) prophylaxis, primarily consisting of CNS-penetrating systemic agents and intrathecal therapy. Although the treatment backbones vary among cooperative groups, the same agents are used, and the outcomes are comparable. ALL therapy typically begins with 5 to 9 months of more-intensive chemotherapy followed by a prolonged low-intensity maintenance phase. Historically, a few cooperative groups treated boys with 1 more year of maintenance therapy than girls; however, most groups treated boys and girls with equal therapy lengths. This practice arose because of inferior survival in boys with older less-intensive regimens. The extra year of therapy added significant burden to patients and families and involved short- and long-term risks that were potentially life threatening and debilitating. The Children’s Oncology Group recently changed its approach as part of its current generation of trials in B-cell ALL and now treats boys and girls with the same duration of therapy. We discuss the rationale behind this change, review the data and differences in practice across cooperative groups, and provide our perspective regarding the length of maintenance therapy.
Introduction and history of therapy for ALL
The improvement in survival for children and young adults with acute lymphoblastic leukemia (ALL) is a remarkable 70-year success story of science and medicine.1 Before the 1950s, ALL was uniformly fatal, with an average survival of 3 months after diagnosis.2 Sidney Farber’s groundbreaking work with aminopterin was the first successful use of a drug to induce remission in human cancer, but all patients relapsed and died.3 In the 1950s and 1960s, the therapeutic landscape changed as cytotoxic agents with activity against ALL were identified and tested as monotherapies, including vincristine and corticosteroids, and as novel agents, including mercaptopurine, were synthesized by pioneers such as Gertrude Elion and George Hitchings.2,4 Unfortunately, although some patients achieved remission, no one was cured by monotherapy.
In the late 1950s, physicians and scientists at the National Cancer Institute, Memorial Sloan Kettering Cancer Center, and Roswell Park Cancer Institute took the next step forward by combining different agents.2 Initial studies treated patients with 2 to 3 drugs over a short period of time, leading to more remissions, but patients inevitably died as a result of the disease. Eventually, in the 1960s and 1970s, some children were cured when Hansjörg (Haig) Riehm in Berlin, Germany, and Donald Pinkel at St Jude Children’s Research Hospital (SJCRH) started trials using multiagent chemotherapy administered in repeated cycles over several years, along with central nervous system (CNS) prophylaxis with cranial irradiation and intrathecal (IT) medications.5,6 Pinkel’s “total therapy” started with a more intensive 3-drug induction, with the goal of achieving remission, followed by an intensification phase of CNS prophylaxis that included cranial irradiation and IT medications, followed by a maintenance phase of less-intensive chemotherapy given over a prolonged period of time. Riehm hypothesized it was crucial to use multiple agents early in therapy and started treatment with a 2-month regimen that included 8 different agents called protocol I, followed by CNS irradiation and a maintenance phase.7 Through consecutively conducted randomized trials, international cooperative groups expanded upon this paradigm, and today, ∼90% of children are cured using modern versions of these approaches.1,8
Currently, a majority of international cooperative groups continue to treat ALL with 4 main phases of therapy over 2 to 3 years (Figure 1). The first phase is a ∼4-week induction phase, including 3 to 4 cytotoxic agents administered in combination with IT chemotherapy, with the goal of obtaining remission, defined classically as <5% bone marrow blasts by morphology. For a majority of groups, this is followed by 3 to 4 months of therapy to consolidate remission, classically including 1 to 2 months of multiagent consolidation chemotherapy with CNS prophylaxis and 2 months of further intensification using a variety of methotrexate-based regimens. The nomenclature differs among cooperative groups; the classic BFM approach, developed by Riehm, terms the first two months induction (IA and IB), followed by a 2-month methotrexate-based consolidation called protocol M.9 After consolidation, patients are treated with a delayed intensification phase, which essentially repeats the drugs given during induction and the first month of consolidation but often substitutes agents for similar ones in the same class (eg, a different corticosteroid, anthracycline, and antimetabolite). This delayed intensification phase, called protocol II in the BFM approach, markedly improves survival.6 Some cooperative groups treat selected patients with a less intensive interim maintenance phase between consolidation and intensification to allow patients to recover from the adverse effects of consolidation before restarting intensive therapy. Over time, interim maintenance has evolved to test different doses and schedules of drugs. Finally, after intensification, patients are treated with a low-intensity maintenance phase, similar to the maintenance developed by SJCRH in the 1960s. CNS-directed therapy remains an integral part of therapy but has transitioned with time to eliminate radiation therapy for most (or all) patients and instead rely on IT and CNS-penetrating systemic chemotherapy.
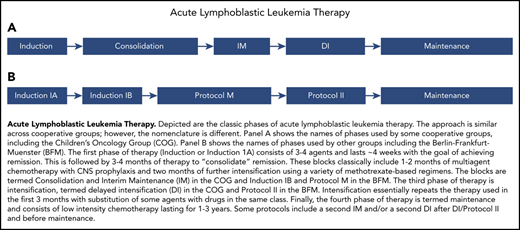
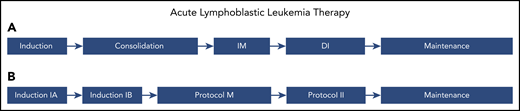
ALL therapy. Classic phases of ALL therapy. The approach is similar across cooperative groups; however, the nomenclature is different. (A) Names of phases used by some cooperative groups, including the Children’s Oncology Group (COG). (B) Names of phases used by other groups, including the Berlin-Frankfurt-Muenster (BFM). The first phase of therapy (induction or induction 1A) consists of 3 to 4 agents and lasts ∼4 weeks, with the goal of achieving remission. This is followed by 3 to 4 months of therapy to consolidate remission. These blocks classically include 1 to 2 months of multiagent chemotherapy with CNS prophylaxis and 2 months of further intensification using a variety of methotrexate-based regimens. The blocks are termed consolidation and interim maintenance (IM) by the COG and induction IB and protocol M by the BFM. The third phase of therapy is intensification, termed delayed intensification (DI) by the COG and protocol II by the BFM. Intensification essentially repeats the therapy used in the first 3 months, with substitution of some agents with drugs in the same class. Finally, the fourth phase of therapy is termed maintenance and consists of low-intensity chemotherapy lasting for 1 to 3 years. Some protocols include a second IM and/or a second DI after DI/protocol II and before maintenance.
ALL therapy. Classic phases of ALL therapy. The approach is similar across cooperative groups; however, the nomenclature is different. (A) Names of phases used by some cooperative groups, including the Children’s Oncology Group (COG). (B) Names of phases used by other groups, including the Berlin-Frankfurt-Muenster (BFM). The first phase of therapy (induction or induction 1A) consists of 3 to 4 agents and lasts ∼4 weeks, with the goal of achieving remission. This is followed by 3 to 4 months of therapy to consolidate remission. These blocks classically include 1 to 2 months of multiagent chemotherapy with CNS prophylaxis and 2 months of further intensification using a variety of methotrexate-based regimens. The blocks are termed consolidation and interim maintenance (IM) by the COG and induction IB and protocol M by the BFM. The third phase of therapy is intensification, termed delayed intensification (DI) by the COG and protocol II by the BFM. Intensification essentially repeats the therapy used in the first 3 months, with substitution of some agents with drugs in the same class. Finally, the fourth phase of therapy is termed maintenance and consists of low-intensity chemotherapy lasting for 1 to 3 years. Some protocols include a second IM and/or a second DI after DI/protocol II and before maintenance.
In a majority of treatment regimens, maintenance chemotherapy consists of daily mercaptopurine and weekly low-dose methotrexate with or without monthly corticosteroid and vincristine pulses and periodic IT chemotherapy. Historically, some groups, including the COG, treated boys with maintenance chemotherapy for an additional year beyond that given to girls. Some cooperative groups administer IT chemotherapy approximately every 3 months during maintenance, which leads to significant disparities in the total number of IT chemotherapy doses given. For example, a 13-year-old girl with favorable disease biology and good response to therapy (minimal residual disease <0.01% at end of induction) treated in the recently completed COG AALL1131 trial would have received 9 more doses of IT chemotherapy than a similar patient treated according to a current BFM protocol (registered at www.clinicaltrials.gov as #NCT03643276), and a boy would have received 13 more doses because of the extra year of maintenance therapy.10 Despite these differences, overall survival (OS) is similar between the different groups, leading to the question of whether there should be sex-based differences in the length of therapy, because the burden of an extra year of chemotherapy is impactful on many levels.8 The importance of the overall length of treatment likely depends on the backbone and intensity of the chemotherapy administered before maintenance. The COG recently changed its approach as part of its current generation of B-cell ALL (B-ALL) trials that opened to accrual in 2019 (AALL1731 [registered at www.clinicaltrials.gov as #NCT03914625], AALL1732 [registered at www.clinicaltrials.gov as #NCT03959085]) and now treats boys and girls with the same duration of therapy. The rationale behind this change and our perspective regarding the length of maintenance therapy comprise the focus of this report. Other aspects of maintenance therapy, including maintenance pulses, intensity of maintenance therapy, and use of IT chemotherapy during maintenance will not be discussed.
Length of maintenance
The COG practice of longer therapy for boys arose from a combined analysis of multiple trials from the Children’s Cancer Study Group from 1968 to 1978.11 The Children’s Cancer Study Group evaluated six trials that included 3161 children with ALL and established boys had modestly inferior survival compared with girls. Early trials (Children’s Cancer Group [CCG]-883 and CCG-903) that did not include CNS prophylaxis showed very poor but equivalent survival in boys and girls; after the inclusion of radiation therapy as part of CNS prophylaxis in subsequent trials, survival improved considerably, and girls fared significantly better.11 In addition to other study questions, children treated in CCG-101, CCG-141, and CCG-143 who were in remission for ∼3 years were randomly assigned to stop therapy or continue to receive therapy for a total duration of 5 years, the standard at the time for the CCG.11-13 For girls, outcomes were equivalent regardless of therapy duration. However, it was unclear if boys had equal outcomes with shorter therapy, because there was a trend toward increased bone marrow and testicular relapse.11 The authors of this meta-analysis noted the results should be interpreted with caution, because the UK Medical Research Council (MRC) had previously reported that boys had a temporary early benefit with longer treatment (3 vs 2 years), but OS was not improved.11,14
Isolated testicular relapse rates remained high (10% to 20%) in the 1970s, and it was hypothesized a subset of boys may have microscopic leukemic infiltrates at the discontinuation of therapy that would predict subsequent testicular relapse.15 Accordingly, in the next generation of CCG trials (CCG-160 series) that enrolled patients from 1978 to 1983, boys were restaged with bilateral testicular biopsy after 8 cycles of maintenance (∼2.5 years after diagnosis).15 Boys with occult testicular leukemia received intensified therapy including testicular irradiation. Boys with negative biopsies were randomly assigned to discontinue therapy or continue therapy for an additional year. Among 1671 boys treated in this series of trials, 5 had testicular involvement at diagnosis. Fewer than 4% of boys (n = 51) experienced testicular relapse during the first 2 years of maintenance. Biopsies were performed for 771 boys (71%), and ∼10% had evidence of occult testicular leukemia. The 4-year event-free survival (EFS) rate for boys who were testicular biopsy negative after 8 cycles of maintenance vs those who were positive was 78.2% ± 4% vs 56% ± 14% (P < .001). Relapse-free survival and OS were also superior in the biopsy-negative group. Only a small percentage of boys (21 of 635) with negative biopsies had testicular relapse as their first event. Moreover, outcomes for patients who had isolated testicular leukemia as the first event after a negative biopsy were similar to the outcomes of those with occult testicular leukemia on biopsy. This led the authors to conclude that biopsy does not change outcome and rather only identifies patients who are likely to relapse earlier in their disease course (lead-time bias). Because ∼10% of patients still had occult disease at this point in therapy, it led to the decision to stop performing end-of-therapy testicular biopsies and to continue treating boys with an additional year of maintenance in subsequent CCG trials, because it did not seem prudent to deescalate therapy. Importantly, systemic chemotherapy was less intensive in this generation of trials; these trials predated modern risk-stratification approaches using prognostic markers including National Cancer Institute risk group, end-induction minimal residual disease, sentinel genetic alterations, and immunophenotype.16
In the same era (1960s to 1980s), several cooperative groups investigated the length of maintenance therapy with randomized trials. Richards et al17 published a comprehensive metaanalysis of 12 000 children with ALL treated in 42 randomized trials performed before 1987. These trials included children treated by the Associazione Italiana di Ematologia Oncologia Pediatrica (AIEOP), BFM, Cancer and Leukemia Group B (CALGB), CCG, French Acute Lymphoblastic Leukemia (FRALLE), Pediatric Oncology Group (POG), UK MRC, and SJCRH cooperative groups. Seventeen of these trials compared different lengths of maintenance, and 16 had available data on 3861 randomly assigned children. For 15 of the 16 trials, randomization occurred when patients reached the point in therapy where the shorter maintenance would end. Three trials (CCG) compared a 5- vs 3-year maintenance duration, 2 trials (BFM) compared 2 years vs 18 months, and the remainder compared 3 vs 2 years. Relapse was more common with shorter maintenance, but this was partially counterbalanced by more deaths resulting from toxicity. Moreover, patients receiving shorter maintenance were more easily salvaged. Thus, there was no difference in OS in the overall cohort or in subanalyses based on age, initial white blood cell count, or sex.
Despite metaanalyses and randomized trials from other groups, most cooperative groups made decisions on length of therapy based on their own historical trials. Table 1 summarizes current and historical differences in length of maintenance therapy across cooperative groups, and Table 2 summarizes the differences in outcome by sex from recent cooperative group trials. Of note, some cooperative groups define length of therapy from diagnosis and others from remission. In North America, current and past cooperative groups include the POG, SJCRH, CALGB, and DFCI, in addition to the CCG. The CALGB made significant contributions to the treatment of childhood leukemia in the 1950s and 1960s, but the CALGB pediatric division was dissolved in 1979 and merged with pediatric members of the Southwest Oncology Group to form the POG.18 The CALGB and POG consistently treated boys and girls with the same length of therapy, recognizing boys had inferior survival.19 When the CCG and POG merged to form the COG in 2001, the CCG therapy was adopted along with the CCG practice of treating boys with 1 extra year of therapy.20 Nevertheless, because of concern for infectious toxicity, children with trisomy 21 have been treated with equal lengths of therapy regardless of sex by the COG since 2009.21
The DFCI initially treated boys and girls with the same duration of therapy.22 In DFCI 87-01, cranial irradiation was eliminated in SR patients as a means of reducing long-term neurocognitive impairment.23 This led to an unacceptable increase in CNS relapses in SR boys but not girls (10-year cumulative incidence of relapse, 20.3% ± 4.6% vs 4.8% ± 2.7%), and the protocol was amended to add an extra year of therapy for SR boys.22 The inclusion of an extra year of therapy for SR boys did not move forward in subsequent trials; however, the DFCI did administer cranial irradiation in more boys than girls in the next trial, DFCI 91-01.24 The DFCI eliminated cranial irradiation for SR boys by intensifying IT therapy based on results from DFCI 95-01.25 Thus, with the exception of a brief window of time in the late 1980s and 1990s, the DFCI has treated boys and girls with similar therapy lengths. SJCRH has treated boys and girls with the same length of therapy, except from 2000 to 2007, when children were treated in Total Therapy XV (TOTXV).26 A primary goal of TOTXV was the elimination of prophylactic cranial irradiation for all patients. A number of changes were made to systemic chemotherapy as compared with preceding trials to eliminate radiation therapy, including treating boys for 6 months longer than girls.26,27 Nevertheless, because the outcomes of boys and girls were equivalent in TOTXV (5-year OS rate, 93.6% ± 2.4% vs 93.3% ± 3.1%; P = .64), equal length of therapy for boys and girls was resumed in Total Therapy XVI, which also demonstrated similar outcomes (5-year OS rate, 94.3% ± 3.1% vs 93.9% ± 3.7%; P = .99).27,28
The BFM investigated 24- vs 18-month treatment duration in ALL-BFM 81 and ALL-BFM 83, finding superior outcomes with 24 months, leading the BFM to adopt a 2-year treatment length for boys and girls as part of an amendment to the ALL-BFM 86 trial.29-32 In ALL-BFM 95, the length of therapy was increased to 36 months for SR boys with B-ALL to prevent late relapses, which were more common in boys than girls in ALL-BFM 90.33 Nevertheless, this intervention did not improve outcomes in SR boys with B-ALL; their outcomes were comparable in ALL-BFM 90 and ALL-BFM 95 and were inferior to girls in both trials. In subsequent trials, starting with AIEOP-BFM 2000, boys and girls with ALL in all risk groups were treated with the same length of therapy.34 Standardizing the length of therapy, including maintenance, made it easier to integrate AIEOP and BFM trials. The AIEOP randomly assigned patients to 3 vs 2 years of total treatment duration in its 1979 series of trials, finding no difference in survival. In 1982, the AIEOP adopted 24 months as the total duration of therapy for all patients (AIEOP study 82).35,36 This practice continued through AIEOP study 95 and the eventual merger of therapy in AIEOP-BFM 2000.29,34,37 Some countries, such as the Czech Republic, did not formally participate in these earlier trials; however, they did treat patients as per the standard arm of the trials and gathered centralized outcome data.38
The Dutch Childhood Leukemia Study Group (DCLSG), later renamed the Dutch Childhood Oncology Group (DCOG), has consistently treated boys and girls with an equal length of therapy.39,40 Early Dutch Childhood Leukemia Study Group trials I to V (1972-1984) used a backbone similar to that of SJCRH.41 Subsequent trials adopted a BFM-like approach when the group merged with the BFM and AIEOP to form the International BFM Study Group in 1986.39 The Nordic Society of Pediatric Hematology and Oncology (NOPHO) was formed in 1981 to gather data from 5 Nordic countries, although treatment did not become uniform until 1992.42 Both before and after the harmonization of therapy, boys and girls were treated with the same therapy length, including maintenance.43 Similarly, the Cooperative Study Group for Childhood Acute Lymphoblastic Leukemia (COALL) has treated boys and girls with an equal therapy duration since the group was formed in 1981.44
The MRC studied duration of therapy in 4 early trials, UK ALL I, II, III, and V, in the 1970s, finding longer maintenance duration was superior in 2 of the trials and inferior in the others.45-48 During this era of trials, the overall outcomes for B-ALL did not improve as compared with outcomes in BFM and CCG studies, leading the MRC to adopt contemporary CCG-like therapy for UK ALL VIII.49 UK ALL VIII (1980-1984) started with 2 years of therapy from remission for boys and girls.50 Shortly after opening, the trial was amended to include a randomization between ∼2 and 3 years of therapy for those patients still in remission after 2 years, finding no benefit with the extra year. The MRC continued to treat boys and girls with an equal duration of therapy until 1999, when it amended the ALL97/99 trial.49,51 The trial was amended to make a number of modifications to standard therapy to mirror treatment used by the CCG at that time (adopted from earlier BFM regimens), including the extra year of maintenance for boys. The practice of treating boys longer than girls continued through the recent UK ALL 2011 trial, which completed accrual in 2017.52
The Children’s Leukemia Cooperative Group (CLCG) from the European Organisation for Research and the Treatment of Cancer (EORTC), consisting of centers in Belgium, France, and Portugal, has treated children with ALL with BFM-like therapy since 1983.53 A large focus of the Children’s Leukemia Cooperative Group–European Organisation for Research and the Treatment of Cancer has been the elimination of cranial irradiation through a series of randomized trials (58831, 58832, and 58881), and it was one of the first cooperative groups to make this major treatment advance.53-55 In these and subsequent trials, boys and girls were treated with similar therapy durations. Similarly, the Israel National Childhood Acute Lymphoblastic Leukemia Study Group (INS) made the elimination of cranial irradiation a major focus of earlier ALL trials in the 1980s and 1990s (INS 84, INS 89, and INS 98).56,57 It was able to make this major advance while treating boys and girls with the same therapy duration.
The FRALLE, recently renamed the Société Française des Cancers et Hémopathies de l’Enfant et de l’Adolescent (SFCE), studied duration of therapy in some of its earlier trials, including FRALLE 83 and FRALLE 87, finding longer maintenance therapy was superior.58,59 Nevertheless, boys and girls have been treated with the same length of therapy. The MRC, which recently changed its name to UKALL, plans to join the Dutch Childhood Oncology Group, Belgian Society of Pediatric Hematology Oncology (BSPHO), Ireland, Société Française des Cancers et Hémopathies de l’Enfant et de l’Adolescent, Cooperative Study Group for Childhood Acute Lymphoblastic Leukemia, and Nordic Society of Pediatric Hematology and Oncology in the recently opened ALLTogether trial (registered at www.clinicaltrials.gov as #NCT03911128), which will treat boys and girls uniformly with a total therapy duration of ∼2 years.39,53
The Japanese Childhood Cancer and Leukemia Study Group (JCCLSG) started treating children with ALL in clinical trials in 1981.60 A number of different maintenance chemotherapy questions were asked in Japanese Childhood Cancer and Leukemia Study Group trials, including ALL811 and ALL814; however, duration of maintenance was not formally studied, and boys and girls were treated with the same type and duration of maintenance therapy.60 In contrast, the Tokyo Children's Cancer Study Group (TCCSG) did investigate a short maintenance regimen in L92-13.61 Historically, children with ALL treated in TCCSG trials received the same duration of therapy regardless of sex. The TCCSG reduced the total duration of therapy from ∼3.5 years for most patients to 2 years between L84-11 and L89-12 clinical trials.62 They successfully did this through a number of changes that included increasing the number of intensive chemotherapy blocks with only 1 year of maintenance. In L92-13, the major study question was to reduce therapy to a total duration of 1 year for all patients.63 To maintain the more intensive blocks, maintenance was reduced to 6 months in SR ALL (age 1-6 years; initial white blood cell count <20 × 109/L, B-cell immunophenotype) and to 3 months for other patients. Relapse rates were high, and boys had worse EFS than in the preceding trial. The 5-year EFS rates of boys and girls were 56.2% ± 3.9% and 71.3% ± 3.6%, respectively. Boys had more salvageable disease, and the 5-year OS rates of boys and girls were 80.5% ± 3.0% and 80.3% ± 3%, respectively. This led the TCCSG to return to ∼2 years of therapy in subsequent trials with an equal therapy length for all.
In 2017, the TCCSG retrospectively performed genomic profiling on banked diagnostic marrow for a subset of patients treated in L92-13 to determine if certain B-ALL patient populations would have good outcomes with 1 total year of therapy.64 It found B-ALL patients with ETV6-RUNX1 (n = 16) and TCF3-PBX (n = 11) had excellent outcomes, with 10-year disease-free survival rates (measured from the end of therapy) of 93.8% ± 6.1% and 90.9% ± 8.7%, respectively. In contrast, patients with high hyperdiploidy (≥52 chromosomes; n = 23) had a poor 10-year disease-free survival rate of 56.6% ± 10.3%. Most relapses in this group were salvageable, because the 12-year OS rate (measured from date of diagnosis) was 91.7% ± −5.6%. These data suggest some B-ALL patients may be cured with short maintenance, and the length of maintenance needed for cure may be driven by disease biology. Future prospective trials are needed.
The Taiwan Pediatric Oncology Group (TPOG), formed in 1988, started performing randomized trials in 1997.65 In these trials (TPOG-ALL-97, TPOG-ALL-2002, and TPOG-ALL-2013), SR and HR boys were treated with a 6-month longer maintenance duration than SR and HR girls (146- vs 120-week total therapy duration).65,66 Very HR boys and girls received more-intensive therapy than those with SR and HR disease, with similar lengths of maintenance (74-week total therapy duration). Outcomes for boys and girls were similar in these trials, and the TPOG continues to treat SR and HR boys longer than girls.
The cumulative data from these groups demonstrate that boys tend to have slightly lower survival than girls, but adding an extra year of maintenance therapy is not an effective means to mitigate this slight survival disadvantage with modern intensive chemotherapy backbones. Although many trials do not have a sufficient number of patients to detect the small differences in outcome, these differences persisted in the 2000 to 2005 era in COG trials, with girls having a 5-year OS rate of 91.0% ± 0.7% and boys of 89.9% ± 0.6% (P = .0213).1 Maintenance therapy, although less intensive, does have risks, because patients can develop life-threatening infections and late effects from prolonged cytotoxic and IT chemotherapy. Even children with SR ALL treated with lower-intensity therapy have increased rates of anxiety and depression and impaired scores for physical, social, and emotional health compared with normative means that persist throughout maintenance therapy.67-69 Daily medication, frequent clinic visits, and additional hospitalizations for infections place burdens on patients, families, and health care systems. These factors are critical to consider in light of the global SARS-CoV-2 pandemic, which may persist for years. The risk of life-threatening infections during maintenance is especially true in low- and middle-income countries. For example, the Brazilian Cooperative Group for Childhood ALL Treatment (GBTLI) recently studied a 6-month reduction in maintenance (18 vs 24 months) in its ALL-93 trial, finding similar outcomes in both groups.70 Twenty-five (3.2%) of 760 patients died as a result of infections during maintenance therapy, with 13 deaths in the 18-month arm and 12 deaths in the 24-month arm. Although infection-related mortality was similar in both arms, this study highlights that patients can and do die as a result of toxicity during maintenance. Recent evidence demonstrates that thiopurine-induced drug resistance mutations facilitate a subset of pediatric ALL relapses, suggesting decreasing exposure might be beneficial by reducing the chance of developing chemotherapy-resistant relapsed clones.71 Prolonging exposure to chemotherapy should only occur if there is a clear benefit.
The ALL therapeutic landscape has changed dramatically with the incorporation of novel immunotherapies into chemotherapy backbones. Multiple immunotherapies have demonstrated efficacy in relapsed and refractory B-ALL in recent trials, including a bispecific T-cell engager that links CD19+ B cells to CD3+ T cells (blinatumomab), an anti-CD22 monoclonal antibody conjugated to calicheamicin (inotuzumab), and multiple CD19-targeting chimeric antigen receptor T cells (CAR-Ts; tisagenlecleucel and axicabtagene ciloleucel).72-75 On the basis of compelling data in relapsed pediatric and adult B-ALL, the COG has moved blinatumomab and inotuzumab into the front line, randomly assigning patients in the aforementioned AALL1731 and AALL1732 trials. Other cooperative groups have also moved these drugs to the front line in AIEOP-BFM ALL 2017 and the aforementioned ALLTogether trial. The COG also incorporated tisagenlecleucel into the frontline treatment of very HR patients in the AALL1721 trial (CASSIOPEIA; registered at www.clinicaltrials.gov as #NCT03876769). In addition, other cooperative groups are opening trials testing immunotherapy in newly diagnosed patients. The goal of maintenance is to use low-intensity chemotherapy to eliminate residual leukemic clones and prevent the emergence of new chemotherapy-resistant clones. With time, immunotherapies may make the need for maintenance obsolete, because they may fulfill the same goals by achieving a deeper molecular remission earlier in therapy. Immunotherapies work by mechanisms different than those of cytotoxic chemotherapy; therefore, they have different selective pressure, which may affect the likelihood of relapse. Indeed, some relapsed B-ALL patients can be cured with CAR-T therapy without any chemotherapy except for pre-CAR-T lymphodepletion. It is more likely that immunotherapies with long persistence, such as CAR-Ts, may affect the duration of therapy necessary for a cure, as compared with immunotherapies with short half-lives. Ultimately, monoclonal antibodies and bispecific T-cell engagers may successfully replace earlier more intensive blocks but may not affect less intensive prolonged maintenance therapy. Future randomized trials are needed to determine if immunotherapies not only improve survival but also allow for the reduction or elimination of chemotherapy.
Length of therapy is not the only difference in maintenance among cooperative groups. Some cooperative groups include repeated pulses of corticosteroids and vincristine and/or IT chemotherapy in maintenance cycles. Similar to maintenance length, any potential benefit of these therapies is largely based on historical data from older trials when therapy was less intensive and outcomes were poor. Early trials that randomly assigned patients to receive or not receive pulses in maintenance found decreased EFS but similar OS rates and no differences in outcome based on sex.17 Some cooperative groups that successfully eliminated the extra year of maintenance in boys without affecting survival did so using a maintenance backbone that included pulses and IT therapy, whereas others eliminated the extra year of maintenance using a backbone that did not include pulses or IT therapy. Currently, outcomes are very similar across cooperative groups regardless of the use of pulses or IT therapy during maintenance. Although it is beyond the scope of this perspective to review these data in detail, they suggest that pulses and IT therapy do not affect the duration of maintenance therapy necessary to obtain cure.
In summary, the choice of whether to treat boys with longer therapy than girls was historically supported by data in an era with less-intensive early therapy, which was associated with significantly lower survival than that obtained with contemporary therapy. With modern intensive risk-stratified therapy, there is no compelling rationale or data to justify treating boys and girls with different therapy durations. The cardinal rule of medicine is “first, do no harm.” Longer therapy clearly causes harm and cannot be supported in the absence of clear evidence of benefit. Future studies should address whether maintenance can be shortened further in certain subsets of ALL based on disease biology. Moreover, there are other differences in maintenance therapy that vary among cooperative groups, such as the use and frequency of vincristine/corticosteroid pulses and IT therapy; whether these are needed is also a subject of substantial debate.
Acknowledgments
This work was supported by National Institutes of Health grants R01CA193776 (D.T.T.), X01HD100702-01 (D.T.T.), U10 CA180886 (M.L.L., D.T.T.), U24 CA196173 (M.L.L.), and 1R01 CA241452 (M.L.L.) from the National Cancer Institute, and P50GM115279 (M.L.L.) from the National Institute of General Medical Sciences; the Leukemia and Lymphoma Society (D.T.T.); Cookies for Kids Cancer (D.T.T.); and the Children’s Oncology Group Foundation (D.T.T.). S.P.H. is the Jeffrey E. Perelman Distinguished Chair in Pediatrics at the Children’s Hospital of Philadelphia. M.L.L. is the Benioff Chair of Children’s Health and the Deborah and Arthur Ablin Endowed Chair for Pediatric Molecular Oncology at Benioff Children’s Hospital.
Authorship
Contribution: D.T.T., S.P.H., and M.L.L. wrote the paper.
Conflict-of-interest disclosure: D.T.T. serves on advisory boards for Janssen, Amgen, La Roche, Sobi, and Humanigen. S.P.H. owns stock in Amgen and has received consulting fees from Novartis and honoraria from Amgen. M.L.L. serves on the advisory board for MediSix Therapeutics.
Correspondence: David T. Teachey, Children’s Hospital of Philadelphia, 3008 CTRB, 3501 Civic Center Blvd, Philadelphia, PA 19104; e-mail: teacheyd@e-mail.chop.edu.