In this issue of Blood, Mikdar et al show that deficiency of ENT1, a nucleoside transporter, leads to defects in erythropoiesis and morphological changes in erythrocytes.1
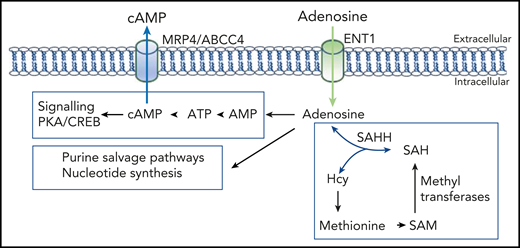
Adenosine levels control multiple biosynthesis pathways. Note that adenosine and homocysteine (Hcy) concentrations coregulate the direction of SAHH. AMP, adenosine monophosphate; CREB, cAMP-response element binding protein; PKA, protein kinase A; SAH, S-adenosyl-l-homocysteine.
Adenosine levels control multiple biosynthesis pathways. Note that adenosine and homocysteine (Hcy) concentrations coregulate the direction of SAHH. AMP, adenosine monophosphate; CREB, cAMP-response element binding protein; PKA, protein kinase A; SAH, S-adenosyl-l-homocysteine.
These phenotypes were linked to adenosine transport and could be rescued, in part, by reduced multidrug transporter MRP4/ABCC4 function. The effects of adenosine were first described as a physiological stimulus that regulates cardiac rhythm.2 Since that study, adenosines have been implicated in the regulation of multiple biological processes. Adenosine transporters can be divided into equilibrative (SLC29) and concentrative (SLC28) transporters. The ENT family of equilibrative transporters uses membrane adenosine concentration gradients as the driving force for influx or efflux. In addition to naturally-occurring nucleosides, nucleotide analogs, such as cytosine arabinoside (ara-C), are substrates. Once intracellular, adenosine metabolism maintains and regulates pivotal biosynthesis pathways, including synthesis of adenosine triphosphate (ATP), cyclic adenosine monophosphate (cAMP), and nucleotides for DNA/RNA synthesis. In addition, through actions of adenosine kinase and S-adenosylhomocysteine hydrolase (SAHH), adenosine regulates S-adenosyl methionine (SAM)‐dependent transmethylation reactions, linking adenosine to epigenetic transcriptional regulation.3 Importantly, adenosine metabolites also provide substrates for an array of other transporters. For instance, efflux of cAMP is mediated by MRP4/ABCC4. Mikdar et al provide evidence that MRP4/ABCC4 fluxes are linked to ENT1 adenosine influx. Taken together, a picture emerges where adenosine/nucleoside transport and metabolism integrate into a large network of biosynthesis pathways, thereby regulating a myriad of biological processes (see figure). Thus, it is not surprising that mutations in such transporters lead to pathology. Mutations in mitochondrial-localized ENT3 lead to familial histiocytosis syndrome,4 whereas ENT1 mutations can result in resistance to chemotherapy (eg, ara-C5 ) or altered binding affinities to specific therapeutics.6 In addition to this, there is considerable interest in the effects of drugs that target these transporters.
ENT1 is also expressed on erythrocytes and harbors the Augustine blood group system, a high-frequency antigen associated with severe hemolytic transfusion reactions.7 Augustine-negative subjects do not display any anemia, but they present with altered bone calcification, indicating a role for ENT1 in bone homeostasis.7 Recently, ENT1 gene regulation during erythropoiesis was shown to be regulated by GATA transcription factors.8 So, what is the role of ENT1 in erythrocytes and during erythropoiesis? Mikdar et al shed new light on this topic. They examined ENT−/− human erythrocytes and found that ENT deficiency leads to macrocytosis and elliptocytosis. Phosphoproteomics uncovered hypophosphorylation of erythrocyte structural proteins that play a role in membrane tethering to the underlying spectrin cytoskeleton (spectrins, protein 4.1, ankyrin, and adducin). Mutations within those proteins lead to elliptocytosis. Surprisingly, no defect in membrane protein tethering to the underlying spectrin cytoskeleton was observed. Although 1 hyperphosphorylated protein, CLNSA1, correlated with macrocytic red cells, the cause of elliptocytosis upon ENT deficiency remains to be elucidated.
Mikdar et al show that ENT1 deficiency reduced human erythrocyte precursor numbers, which resulted in anemia and macrocytosis in Ent1−/− mice. These defects could be overcome, in part, by reduced activity of the multidrug transporter MRP4/ABCC4, observed upon inhibition with the broad MRP inhibitor MK-571 or in ENT−/− individuals with an additional heterozygous loss-of-function mutation in MRP4. Strikingly, wild-type erythropoiesis was also enhanced in mice treated with ABCC4/MRP4 inhibitors. The results show that erythropoiesis is controlled, in part, by nucleoside transporters and nucleotide exporters; importantly, this pathway may be exploited for therapeutic purposes to treat anemias. However, because complete ENT1-null individuals do not suffer from anemia or from reduced platelet or white blood cell counts, the usability of this MRP4 inhibitor remains unclear with respect to other erythroid pathology or, in general, the hematopoietic system, a focal point for future research. Of note, MK-571 was clinically tested for treatment of asthma, pulmonary arterial hypertension, and platelet aggregation.9 It would be interesting to monitor erythroid parameters in these patients. Still, it remains an open question why the ENT1−/− and ENT1-knockdown subjects present with a severe in vitro erythroid defect, whereas ENT-deficient individuals do not present with anemia. Interestingly, Ent−/− mice are capable of splenic stress erythropoiesis, suggesting that proliferation of these committed erythroblasts is unaffected. So, when do ENT1 and MRP4 elicit their effects during erythropoiesis? It is tempting to speculate that, in vivo, decreased erythroid specification is negated by increased expansion of committed erythroblasts as a compensatory mechanism. In agreement with this, Mikdar et al show reduced frequencies of nonerythroid committed megakaryocyte erythroid progenitors and common myeloid progenitors. The underlying mechanisms may not be fully recapitulated in vitro, resulting in the observed decreased erythroid output. In spite of this, the data suggest that adenosine homeostasis plays a role in erythropoiesis before the committed erythroblast stage.
What are the mechanisms that drive these effects on erythropoiesis? Adenosine treatment during erythropoiesis led to increased levels of cyclic nucleotides and enhanced terminal differentiation that was controlled by ENT1. Although it is reasonable to assume that MRP4/ABCC4 inhibition would result in accumulation of intracellular cAMP/guanosine 3′,5′-cyclic monophosphate during ENT−/− erythropoiesis, thus leading to the observed rescue of erythropoiesis, this has not been shown. However, Mikdar et al observed an interesting link between adenosine treatment and increased cAMP-dependent protein kinase A activation and cAMP-response element binding protein phosphorylation. This might regulate specific transcriptional programs affecting erythropoiesis. It would be interesting to understand the specifics of these transcriptional programs and how MRP4/ABCC4 inhibitors or ENT1 deficiency affect these. Combining this with the modulation of SAM‐dependent transmethylation by adenosine levels, which regulates transcriptional regulation of gene expression through epigenetic control, merits a closer look at the relationship between adenosine availability and global methylation patterns during erythropoiesis. Indeed, global genome methylation patterns are highly regulated at the onset of erythroblast differentiation.10
In conclusion, Mikdar et al studied the effects of nucleoside transport function by ENT1 and unraveled novel pathways that regulate erythropoiesis. These might be therapeutically targeted to treat anemia. Because of the clinical interest in ENT and MRP transporters, from multidrug responses to transport of nucleotide analogs, the effects of specific agonists and antagonists on hematopoiesis are important to evaluate.
Conflict-of-interest disclosure: E.v.d.A. declares no competing financial interests.