In this issue of Blood,Goshua et al present a timely cost-effectiveness analysis of caplacizumab for immune thrombotic thrombocytopenic purpura (iTTP) and conclude that adding caplacizumab to standard-of-care (SOC) therapy with plasma exchange and immunosuppression is not cost-effective based on outcomes including duration of hospitalization and iTTP recurrence.1
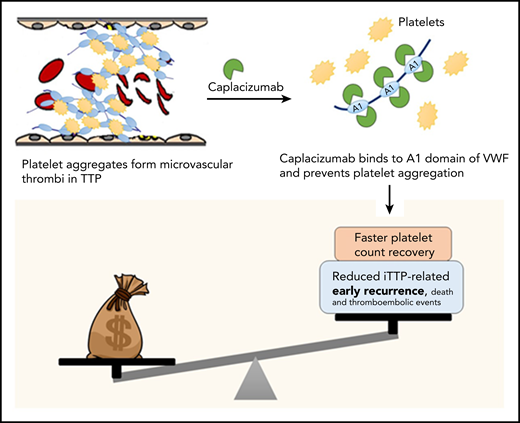
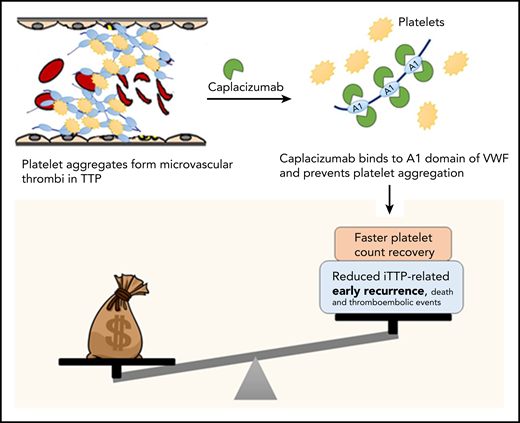
Costs vs benefits of caplacizumab for TTP. In TTP, platelets form aggregates by binding to ultra-large multimers of VWF; these aggregate form microthrombi that lead to ischemic organ damage. Caplacizumab is a nanobody directed against domain A1 of VWF, which prevents VWF-platelet interactions, leading to faster resolution of thrombocytopenia in TTP. In clinical trials, adding caplacizumab to standard-of-care therapy led to faster time to platelet count recovery (and reduced duration of plasma exchange and hospitalization, as well as a reduction in the composite endpoint of iTTP-related death, recurrence, and major thromboembolic events, which was driven primarily by the reduction in early recurrences). However, the current analysis demonstrates that caplacizumab is not cost effective at its current list price in the United States.
Costs vs benefits of caplacizumab for TTP. In TTP, platelets form aggregates by binding to ultra-large multimers of VWF; these aggregate form microthrombi that lead to ischemic organ damage. Caplacizumab is a nanobody directed against domain A1 of VWF, which prevents VWF-platelet interactions, leading to faster resolution of thrombocytopenia in TTP. In clinical trials, adding caplacizumab to standard-of-care therapy led to faster time to platelet count recovery (and reduced duration of plasma exchange and hospitalization, as well as a reduction in the composite endpoint of iTTP-related death, recurrence, and major thromboembolic events, which was driven primarily by the reduction in early recurrences). However, the current analysis demonstrates that caplacizumab is not cost effective at its current list price in the United States.
Caplacizumab is a first-in-class nanobody directed against domain A1 of von Willebrand factor, which prevents platelet aggregation because of platelet-von Willebrand factor interactions.2,3 Caplacizumab is the first therapy to receive US Food and Drug Administration approval for the treatment of iTTP in combination with plasma exchange and immunosuppression based on the results of the phase 3 Trial With Caplacizumab in Patients With Acquired Thrombotic Thrombocytopenic Purpura (HERCULES) trial, which showed that adding caplacizumab led to faster resolution of thrombocytopenia and a 74% reduction in the composite end point of iTTP-related death, recurrence and major thromboembolic events, and somewhat reduced time to normalization of markers of end-organ damage.2 There were no cases of refractory iTTP or deaths in the caplacizumab arm (4% each with placebo). Although caplacizumab shortens duration of plasma exchange and hospitalization, it does not address the underlying immunopathology of iTTP because it does not address the ADAMTS13 inhibitory autoantibody. Despite the fact that caplacizumab has demonstrated efficacy in treating TTP and is conditionally recommended by the International Society on Thrombosis and Haemostasis iTTP guidelines,4 cost remains a major barrier to its widespread use. At ∼$7700 for a single dose, an average course of therapy costs ∼$270 000. Combined with the fact that most patients will recover from their acute episode of TTP with plasma exchange and immunosuppression, many hospitals and clinicians have been reluctant to incorporate caplacizumab as part of frontline therapy for all iTTP.
In this analysis, the authors used data regarding probability of relapse and death from the phase 2 Study to Assess Efficacy and Safety of Anti–von Willebrand Factor (vWF) Nanobody in Patients With Acquired TTP (TITAN) and HERCULES trials to evaluate cost effectiveness from the point of view of the health care system. The incremental cost effectiveness ratio of adding caplacizumab to SOC, which is the additional cost per quality adjusted life-year gained, was >$1.4 million, which is significantly higher than the $195 300 willingness to pay threshold in the United States. These results held true despite the author’s attempts to minimize bias against caplacizumab by minimizing baseline estimates of recurrence with caplacizumab and maximizing estimated relapse rates with SOC. In a sensitivity analysis, the authors concluded that decreasing the cost of caplacizumab (vs changing factors such as cost of rituximab, and duration of plasma exchange, hospitalization, or intensive care stay) would have the greatest impact on cost-effectiveness.
Caplacizumab does not appear to be cost-effective as currently used, but represents an important advance in iTTP therapy, and clearly improves short-term outcomes including reducing (and possibly eliminating) refractoriness to plasma exchange and mortality in iTTP, both of which are unpredictable.5 Real-world data are required to establish which patients are most likely to benefit from caplacizumab that may be, as the authors suggest, patients that are refractory to plasma exchange or present with cardiac or neurologic symptoms predictive of mortality.5 However, based on current data, the greatest benefit is likely to be seen when caplacizumab is used early in the treatment course. Additionally, the ideal duration of caplacizumab therapy has not been established. In HERCULES, caplacizumab was continued for at least 30 days after stopping plasma exchange, but many experts agree that it is likely safe to discontinue caplacizumab after ADAMTS13 activity recovers to >10% to 20%.6 In a recent report of 60 patients from Germany that used ADAMTS13 activity to guide duration of caplacizumab therapy, 58.3% of patients required treatment for less than 30 days, resulting in cost savings of €2.49 million (or $3.4 million).7 Caplacizumab use is likely to increase early use of intensive immunosuppression including rituximab and require increased ADAMTS13 monitoring, which will offset some of these cost savings. Finally, the impact, if any, of caplacizumab on long-term outcomes of iTTP is unknown. iTTP survivors experience a number of adverse health outcomes including neurocognitive impairment, stroke, and reduced quality of life in addition to shortened survival compared with age- and sex-matched controls.8 It is plausible that the microvascular injury during acute iTTP episodes contributes to these long-term sequelae especially neurocognitive deficits. Additional studies are needed to evaluate whether caplacizumab mitigates these effects, which would impact the cost-benefit analysis (see figure).
The broader issue of the high cost of prescription drugs, and orphan drugs in particular, threatens health care budgets and presents unaffordable out-of-pocket costs in countries without universal health care. This challenge is not limited to caplacizumab and therapies for other rare disorders such as paroxysmal nocturnal hemoglobinuria and lysosomal storage disorders have incremental cost effectiveness ratios that are >$1 million but the benefit of these agents that improve outcomes for patients with serious illnesses is not in question. The high cost of innovation and drug development is one of the factors driving high costs for novel therapies. Other systemic factors contributing to high drug costs include the existence of monopolies, slow development and approval of generics and biosimilars, and the lack of negotiation over drug prices, particularly in the United States, where the cost of prescription drugs is often several times higher than in other developed countries.9,10 Addressing these issues will require policy changes that favor transparency in pricing, systems to negotiate value based pricing of new drugs based on incremental benefit as well as accounting for the cost of innovation, and enhancing programs that assist patients with out-of-pocket drug costs. Physician and patient organizations play an important advocacy role to catalyze these changes.
Since the landmark trial establishing plasma exchange as the standard of care for iTTP 30 years ago, caplacizumab is the first therapy to demonstrate efficacy in a randomized clinical trial in iTTP, and other novel agents including recombinant ADAMTS13 are in clinical trials. Future studies will ideally incorporate longer term outcomes such as neurocognitive function and other patient-reported outcomes, and reduced or no dependence on plasma therapy to revolutionize TTP outcomes. Until then, real-world data are needed to inform the optimal, and most cost-effective, ways to incorporate novel targeted agents into current treatment paradigms.
Conflict-of-interest disclosure: S.C. has served on advisory boards for Sanofi-Genzyme, Alexion, and Takeda.